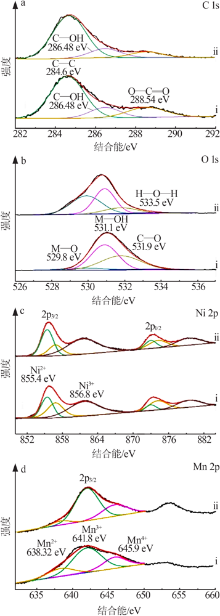
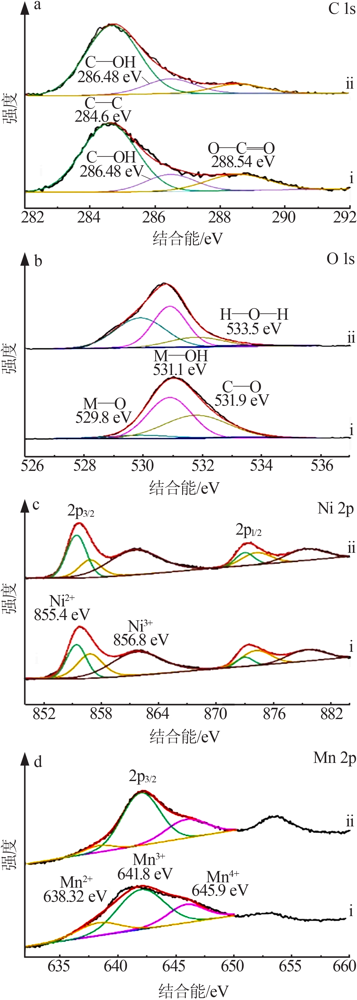
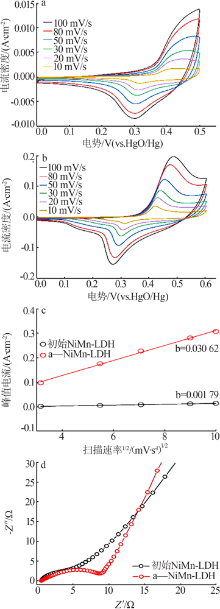
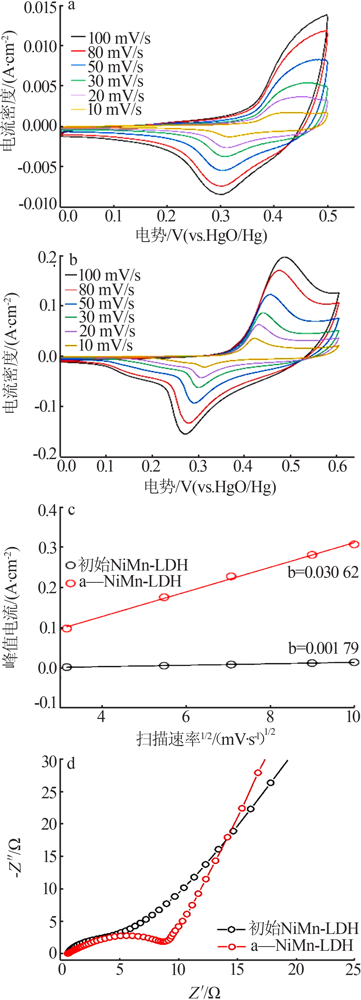
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (2): 38-44.doi: 10.19964/j.issn.1006-4990.2021-0223
• Research & Development • Previous Articles Next Articles
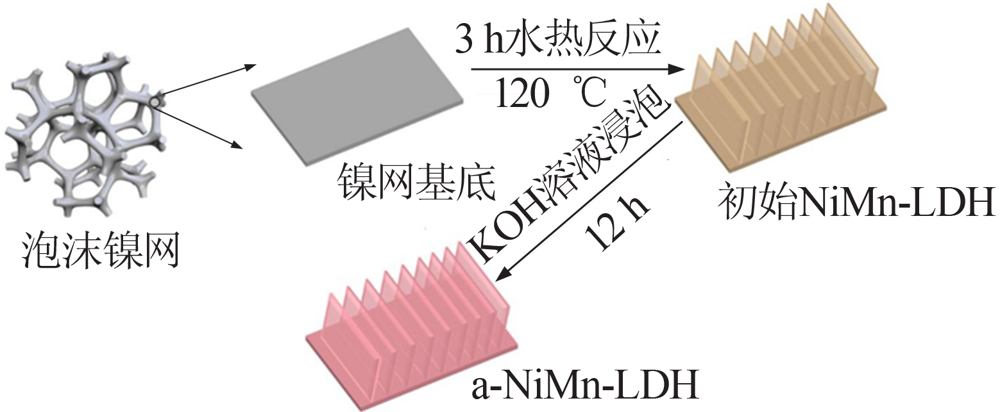
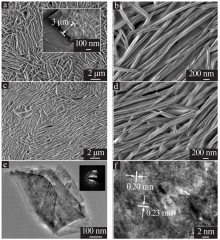
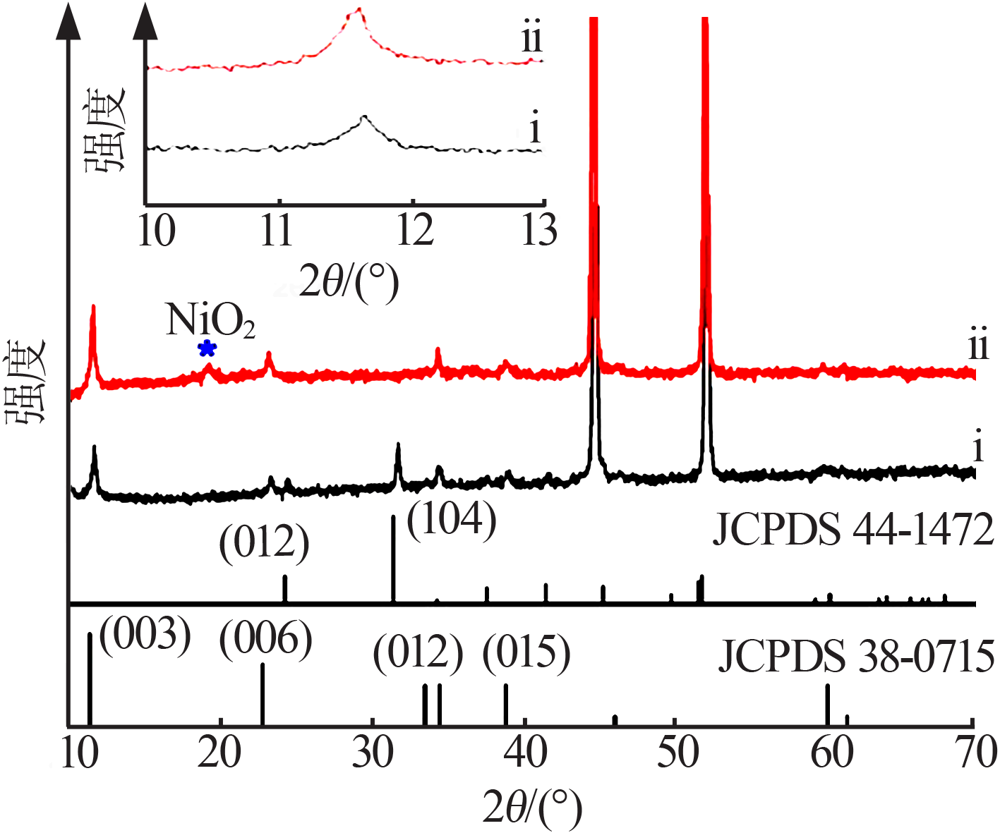
Study on improvement of capacitance performance of NiMn-LDH electrode material by anions exchange
LIANG Qunfang( ),XU Xuetang(
),XU Xuetang( ),WANG Fan
),WANG Fan
- School of Chemistry and Chemical Engineering,Guangxi University,Nanning 530004,China
-
Received:2021-04-06Online:2022-02-10Published:2022-03-14 -
Contact:XU Xuetang E-mail:1339247473@qq.com;xxtang@gxu.edu.cn
CLC Number:
Cite this article
LIANG Qunfang,XU Xuetang,WANG Fan. Study on improvement of capacitance performance of NiMn-LDH electrode material by anions exchange[J]. Inorganic Chemicals Industry, 2022, 54(2): 38-44.
share this article
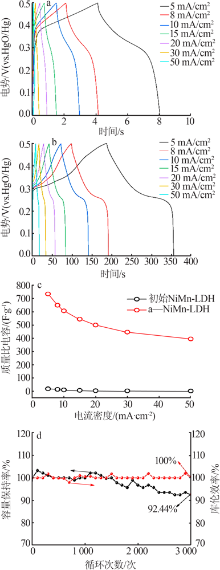
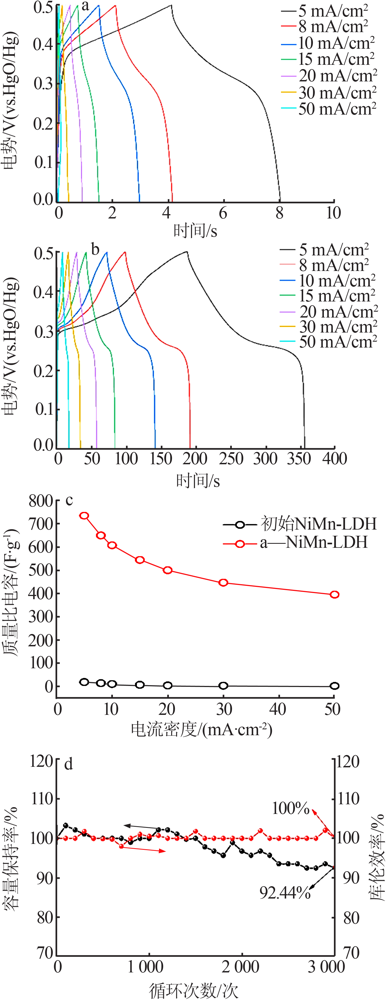
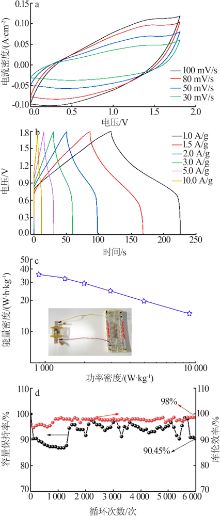
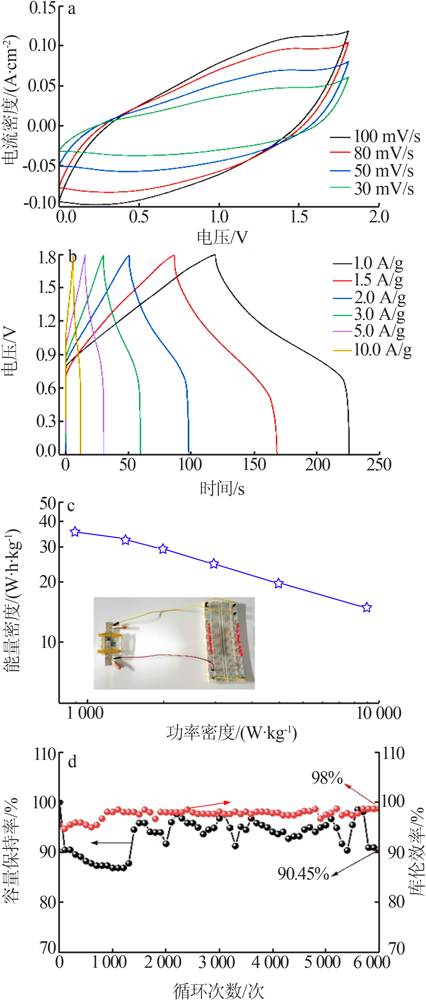
Table 1
Comparison of the electrochemical performance of various NiMn-based all-solid-state supercapacitors"
| 正极 | 负极 | 能量密度/ (W·h·kg-1) | 功率密度/ (W·kg-1) |
|---|---|---|---|
| a-NiMn LDH | AC | 35.9 | 900.00 |
| NiMn-LDH/NCF[ | NCF | 34.1 | 350.10 |
| NiMn-LDH/PC-1[ | RGO/CNT | 18.6 | 225.03 |
| NiMn-LDH/PPy/BC[ | Fe3O4@C/MWCNTs | 29.8 | 299.00 |
| rGO@NiMn-LDH@NF[ | AC | 22.5 | 700.00 |
| carbon-NiMn-LDH/NF[ | AC | 37.7 | 378.13 |
| [1] |
NGUYEN T, MONTEMOR M. Metal oxide and hydroxide-based aqueous supercapacitors:From charge storage mechanisms and functional electrode engineering to need-tailored devices[J]. Adva-nced Science, 2019, 6.Doi: 10.1002/advs.201801797.
doi: 10.1002/advs.201801797 |
| [2] | 张硕嘉, 杨玉彬, 唐宇, 等. 高活性Fe2O3@Ni复合电极制备及电化学性能研究[J]. 无机盐工业, 2019, 51(7):24-27. |
| [3] |
CHOUDHARY N, LI C, MOORE J, et al. Supercapacitors:Asymme-tric supercapacitor electrodes and devices[J]. Advanced Materials, 2017, 29.Doi: 10.1002/adma.201605336.
doi: 10.1002/adma.201605336 |
| [4] | 赵杰, 郭月, 沈桢, 等. 高倍率容量层状双金属氢氧化物超级电容材料的研究进展[J]. 化工学报, 2020, 71(11):4851-4872. |
| [5] | 王文聪. 层状双金属氢氧化物超级电容器电极材料的制备和电化学性能研究[D]. 杭州:浙江大学, 2019. |
| [6] |
WANG R, YAO M, NIU Z. Smart supercapacitors from materials to devices[J]. InfoMat, 2020, 2:113-125.
doi: 10.1002/inf2.v2.1 |
| [7] | 朱基亮. 超级电容器用金属层状双氢氧化物电极材料研究进展[J]. 四川师范大学学报:自然科学版, 2020, 43(3):285-296. |
| [8] | 雷娜, 马翩翩. Ni-Co LDHs纳米片阵列用于超级电容器电极的研究[J]. 浙江理工大学学报:自然科学版, 2020, 43(6):58-64. |
| [9] |
XU R, DU L, ADEKOYA D, et al. Well-defined nanostructures for electrochemical energy conversion and storage[J]. Advanced Ener-gy Materials, 2020, 10.Doi: 10.1002/aenm.202001537.
doi: 10.1002/aenm.202001537 |
| [10] | 李宫, 陈昆峰, 金京一, 等. La3+掺杂NiCo层状双金属氢氧化物纳米片的合成及其电化学性能[J]. 应用化学, 2017(34):71-75. |
| [11] |
LIU H B, LI J C, YE L, et al. Co1-x-yNixZny(CO3)0.5(OH)·0.11H2O nanoneedles-NiCo-layered double hydroxide nanosheet compos-ites on vulcanized Ni foams for supercapacitors[J]. ACS Applied Nano Materials, 2021, 4(2):1743-1753.
doi: 10.1021/acsanm.0c03200 |
| [12] |
WANG Y, YAN D, EL HANKARI S, et al. Recent progress on lay-ered double hydroxides and their derivatives for electrocatalytic water splitting[J]. Advanced Science, 2018, 5(8).Doi: 10.1002/advs.201800064.
doi: 10.1002/advs.201800064 |
| [13] |
WANG X L, ZHANG J Q, YANG S B, et al. Interlayer space regu- lating of NiMn layered double hydroxides for supercapacitors by controlling hydrothermal reaction time[J]. Electrochimica Acta, 2019, 295:1-6.
doi: 10.1016/j.electacta.2018.10.021 |
| [14] |
TANG Y Q, SHEN H M, CHENG J Q, et al. Fabrication of oxygen-vacancy abundant NiMn-layered double hydroxides for ultrahigh capacity supercapacitors[J]. Advanced Functional Materials, 2020, 30.Doi: 10.1002/adfm.201908223.
doi: 10.1002/adfm.201908223 |
| [15] | 邹文茹. 阴离子交换法增强Ni-Co基纳米阵列电极性能的研究[D]. 南宁:广西大学, 2019. |
| [16] |
YANG T Y, YE J, CHEN S H, et al. Construction of nanowall-su-pported-nanorod nico ldh array electrode with high mass-loadingon carbon cloth for high-performance asymmetric supercapacitors[J]. Electrochimica Acta, 2020, 362.Doi: 10.1016/j.electacta.2020.137081.
doi: 10.1016/j.electacta.2020.137081 |
| [17] |
ZANG Y, LUO H, ZHANG H, et al. Polypyrrole nanotube-interco-nnected NiCo-LDH nanocages derived by ZIF-67 for supercapac-itors[J]. ACS Applied Energy Materials, 2021, 4(2):1189-1198.
doi: 10.1021/acsaem.0c02465 |
| [18] | LIU X, ZHOU A, PAN T, et al. Ultrahigh-rate-capability of a layered double hydroxide supercapacitor based on a self-generated elec-trolyte reservoir[J]. Journal of Materials Chemistry A, 2016(4):8421-8427. |
| [19] |
WEI M, MA R, WU J, et al. Development of efficient electrocataly-sts via molecular hybridization of NiMn layered double hydroxide nanosheets and graphene[J]. Nanoscale, 2016, 8(19):10425-10432.
doi: 10.1039/C6NR00988C |
| [20] |
CHEN D M, YAN S, CHEN H J, et al. Hierarchical Ni-Mn layered double hydroxide grown on nitrogen-doped carbon foams as high-performance supercapacitor electrode[J]. Electrochimica Acta, 2018, 292:374-382.
doi: 10.1016/j.electacta.2018.07.042 |
| [21] |
YU M, LIU R L, LIU J H, et al. Polyhedral-like NiMn-layered dou-ble hydroxide/porous carbon as electrode for enhanced electroche-mical performance supercapacitors[J]. Small, 2017, 13(44).Doi: 10.1002/smll.201702616.
doi: 10.1002/smll.201702616 |
| [22] |
CHEN H Y, AI Y N, LIU F, et al. Carbon-coated hierarchical Ni-Mn layered double hydroxide nanoarrays on Ni foam for flexible high-capacitance supercapacitors[J]. Electrochimica Acta, 2016, 213:55-65.
doi: 10.1016/j.electacta.2016.06.038 |
| [23] |
SUN L, ZHANG Y X, ZHANG Y, et al. Reduced graphene oxide nanosheet modified NiMn-LDH nanoflake arrays for high-perfor-mance supercapacitors[J]. Chemical Communications, 2018, 54(72):10172-10175.
doi: 10.1039/C8CC05745A |
| [24] |
XU T, LI G, YANG X, et al. Design of the seamless integrated C@NiMn-OH-Ni3S2/Ni foam advanced electrode for supercapaci-tors[J]. Chemical Engineering Journal, 2019, 362:783-793.
doi: 10.1016/j.cej.2019.01.083 |
| [1] | ZHANG Feigang, LIU Zhongli. Study on application of CuO/g-C3N4 composites in organic dye degradation and supercapacitors [J]. Inorganic Chemicals Industry, 2025, 57(1): 129-136. |
| [2] | WU Qingqing, XU Xuetang, WANG Fan. Study on capacitor performance of high⁃mass⁃loading ZnCo-based carbonate hydroxide electrode materials [J]. Inorganic Chemicals Industry, 2024, 56(7): 46-54. |
| [3] | WANG Jianfang, YANG Heping, LI Kaibin, CONG Shiqiang, ZHANG Bojie, GUO Shan. Study on preparation of C3N4/MnCo2S4 composites and their capacitive properties [J]. Inorganic Chemicals Industry, 2023, 55(7): 70-74. |
| [4] | XU Xuetang, WANG Xukai, ZHANG Shenhe, HUANG Meixiang, NONG Shuliu, XU Nuo. Study on preparation and properties of vanadate doped NiCo-LDH electrode [J]. Inorganic Chemicals Industry, 2023, 55(5): 52-58. |
| [5] | JIANG Tiantian,XU Xuetang,WANG Fan. Research on growth and supercapacitance of NiCo based electrode materials regulated by halogen ions [J]. Inorganic Chemicals Industry, 2022, 54(8): 66-73. |
| [6] | Zhang Tianliang,Li Jun,Xiong Wei,Zhang Haiyan,Tao Xiaoqiu. Study on one-step preparation of activated carbon with high specific surface by K2CO3 activation and its capacitance performance [J]. Inorganic Chemicals Industry, 2022, 54(4): 159-164. |
| [7] | WANG Dian,SU Qiong,PANG Shaofeng,CAO Shijun,KANG Lihui,LIANG Lichun,WANG Yanbin,LI Zhaoxia. Study on high-performance supercapacitors based on Fe2O3/biomass carbon composites [J]. Inorganic Chemicals Industry, 2022, 54(3): 59-65. |
| [8] | ZHAO Zhichao,WANG Honglin,WANG Xia,SUN Gang,ZHAO Cuilian,SUN Nannan. Controllable preparation of NiMoO4 nanosheets-based microspheres by hydrothermal method and their supercapacitor properties [J]. Inorganic Chemicals Industry, 2022, 54(2): 60-64. |
| [9] | YI Jinliang,YANG Min,SONG Fangxiang,CHEN Qianlin. Study on preparation and electrochemical properties of agaric carbon-based cobalt sulfide composites [J]. Inorganic Chemicals Industry, 2022, 54(12): 60-67. |
| [10] | Shen Wei,Wang Sinan,Liang Xuemei,Wei Jinyun,Pan Yujie,Nong Tiantian,Zhou Yan,Tan Xuecai,Huang Zaiyin. Research progress of nano MOFs and their derivatives for supercapacitors [J]. Inorganic Chemicals Industry, 2021, 53(6): 79-86. |
| [11] | Wang Yuexiang,Shao Lanyan,Xu Tiannan,Cai Caihong,Yuan Junsheng,Zhang Yingwu. Study on existing speciation and deep dechlorination of chlorine in waste incineration fly ash [J]. Inorganic Chemicals Industry, 2021, 53(5): 78-83. |
| [12] | Zhang Hao,Wang Liyan,Li Yingqi,Xiao Shanshan,Bi Fei,Zhao Li. Nano-flowered ZnCo LDHs electrode material grown in situ on nickel foam and its electrochemical properties [J]. Inorganic Chemicals Industry, 2021, 53(5): 61-65. |
| [13] | Zhang Shuojia,Yang Yubin,Tang Yu,Chi Liping,Xu Bing. Synthesis and electrochemical properties of high?鄄activity Fe2O3@Ni composite electrode [J]. Inorganic Chemicals Industry, 2019, 51(7): 24-27. |
| [14] | Zhao Yu,Zhang Shuojia,Xu Bing,Yu Yue,Sun Xiaohui. Fabrication and electrochemical properties of Zn-based electrode materials [J]. Inorganic Chemicals Industry, 2019, 51(6): 21-24. |
| [15] | Luo Xiqing,Wang Tongzhen,Jiang Miaomiao,Xiong Fan,Cheng Fengru. Study on preparation of NiS2/Ni(OH)2 hollow spheres and its capacitor performance [J]. Inorganic Chemicals Industry, 2019, 51(12): 35-38. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||