Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (11): 10-16.doi: 10.19964/j.issn.1006-4990.2020-0666
• Reviews and Special Topics • Previous Articles Next Articles
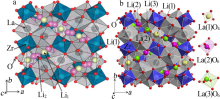
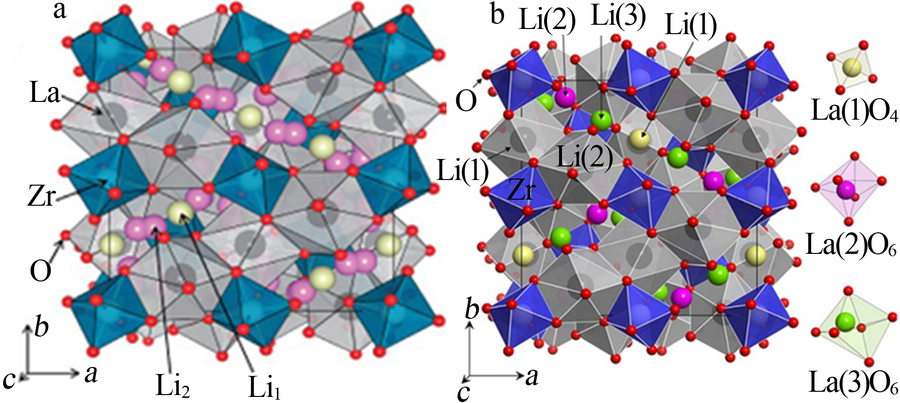
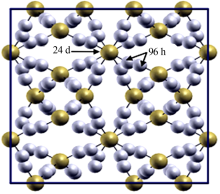
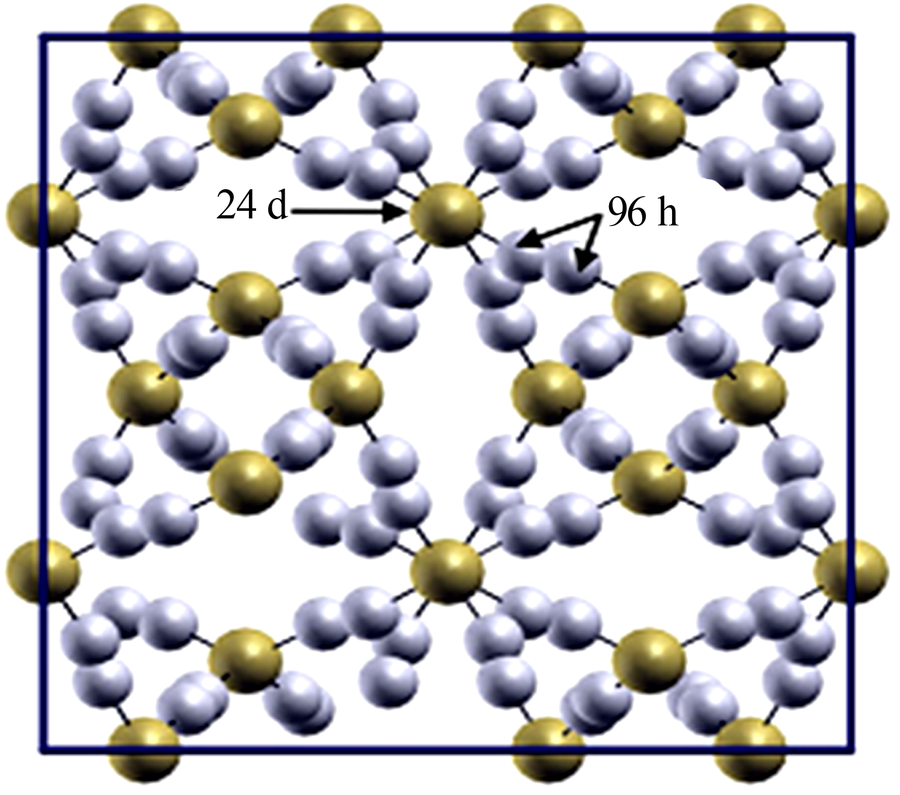
Research progress on modification of solid inorganic electrolyte of Li7La3Zr2O12
LU Chao( ),LI Mingming,WU Xiaoqiang,AN Xuguang,KONG Qingquan,WANG Xiaolian
),LI Mingming,WU Xiaoqiang,AN Xuguang,KONG Qingquan,WANG Xiaolian
- School of Mechanical Engineering,Chengdu University,Chengdu 610106,China
-
Received:2020-12-09Online:2021-11-10Published:2021-11-15
CLC Number:
Cite this article
LU Chao,LI Mingming,WU Xiaoqiang,AN Xuguang,KONG Qingquan,WANG Xiaolian. Research progress on modification of solid inorganic electrolyte of Li7La3Zr2O12[J]. Inorganic Chemicals Industry, 2021, 53(11): 10-16.
share this article
| [1] |
BI K, ZHAO S X, HUANG C, et al. Improving low-temperature per-formance of spinel LiNi0.5Mn1.5O4 electrode and LiNi0.5Mn1.5O4/Li4Ti5O12 full-cell by coating solid-state electrolyte Li-Al-Ti-P-O[J]. Journal of Power Sources, 2018, 389:240-248.
doi: 10.1016/j.jpowsour.2018.03.071 |
| [2] |
LI W, YAO H, YAN K, et al. The synergetic effect of lithium poly-sulfide and lithium nitrate to prevent lithium dendrite growth[J]. Nat Commun, 2015, 6.Doi: org/10.1038/ncomms8436.
doi: org/10.1038/ncomms8436 |
| [3] | LIU G, XIE D, WANG X, et al. High air-stability and superior lithi-um ion conduction of Li3+3xP1-xZnxS4-xOx by aliovalent substitution of ZnO for all-solid-state lithium batteries[J]. Energy Storage Materi-als, 2019, 17:266-274. |
| [4] |
RAMASWAMY M, VENKATARAMAN T, WERNER W. Fast lithi-um ion conduction in garnet-type Li7La3Zr2O12[J]. Angew Chem Int Ed Engl, 2007, 46:7778-7781.
doi: 10.1002/(ISSN)1521-3773 |
| [5] |
AWAKA J, KIJIMA N, HAYAKAWA H, et al. Synjournal and struc-ture analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure[J]. Journal of Solid State Chemistry, 2009, 182(8):2046-2052.
doi: 10.1016/j.jssc.2009.05.020 |
| [6] |
LIU Q, GENG Z, HAN C, et al. Challenges and perspectives of gar-net solid electrolytes for all solid-state lithium batteries[J]. Journal of Power Sources, 2018, 389:120-134.
doi: 10.1016/j.jpowsour.2018.04.019 |
| [7] |
BERNSTEIN N JOHANNES M D; HOANG K. Origin of the struc-tural phase transition in Li7La3Zr2O12[J]. Phys Rev Lett, 2012, 109(20).Doi: org/10.1103/PhysRevLett.109.205702.
doi: org/10.1103/PhysRevLett.109.205702 |
| [8] | MUKHOPADHYAY S, THOMPSON T, SAKAMOTO J, et al. Struc-ture and stoichiometry in supervalent doped Li7La3Zr2O12[J]. Chemi-stry of Materials, 2015, 27(10):3658-3665. |
| [9] |
XIE H, ALONSO J A, LI Y, et al. Lithium distribution in aluminum-free cubic Li7La3Zr2O12[J]. Chemistry of Materials, 2011, 23(16):3587-3589.
doi: 10.1021/cm201671k |
| [10] |
DÜVEL A, KUHN A, ROBBEN L, et al. Mechanosynjournal of solid electrolytes:Preparation,characterization,and Li ion transport pro-perties of garnet-type Al-Doped Li7La3Zr2O12 crystallizing with cubic symmetry[J]. The Journal of Physical Chemistry C, 2012, 116(29):15192-15202.
doi: 10.1021/jp301193r |
| [11] |
WU J F, CHEN E Y, YU Y, et al. Gallium-doped Li7La3Zr2O12 gar-net-type electrolytes with high lithium-ion conductivity[J]. ACS Appl Mater Interfaces, 2017, 9(2):1542-1552.
doi: 10.1021/acsami.6b13902 |
| [12] |
WAGNER R, REDHAMMER G J, RETTENWANDER D, et al. Fast Li-ion-conducting garnet-related Li7-3xFexLa3Zr2O12 with un-common I43d structure[J]. Chem Mater, 2016, 28(16):5943-5951.
doi: 10.1021/acs.chemmater.6b02516 |
| [13] | 吴润平, 朱琳, 洪涛, 等. 锌掺杂Li7La3Zr2O12 固体电解质材料的制备及电化学性能研究[J]. 冶金与材料, 2019, 39(3):1-3. |
| [14] | 查文平, 李君阳, 阳敦杰, 等. 无机固体电解质Li7La3Zr2O12 的研究进展[J]. 中国材料进展, 2017, 36(10):700-707. |
| [15] |
BUSCHMANN H, BERENDTS S, MOGWITZ B, et al. Lithium metal electrode kinetics and ionic conductivity of the solid lithium ion conductors “Li7La3Zr2O12” and Li7-xLa3Zr2-xTaxO12 with garnet-type structure[J]. Journal of Power Sources, 2012, 206:236-244.
doi: 10.1016/j.jpowsour.2012.01.094 |
| [16] |
CAO Z Z, REN W, LIU J R, et al. Microstructure and ionic conduc-tivity of Sb-doped Li7La3Zr2O12 ceramics[J]. Journal of Inorganic Materials, 2014, 29(2):220-224.
doi: 10.3724/SP.J.1077.2013.13428 |
| [17] | 李雪妍, 罗亚历, 陈涵, 等. 掺杂量及烧结温度对W掺杂Li7La3Zr2O12 陶瓷电解质性能的影响[J]. 南京工业大学学报:自然科学版, 2020, 42(1):81-86. |
| [18] |
RETTENWANDER D, WELZL A, CHENG L, et al. Synjournal,cry-stal chemistry,and electrochemical properties of Li7-2xLa3Zr2-xMoxO12 (x=0.1~0.4):Stabilization of the cubic garnet polymorph via subs-titution of Zr4+ by Mo6+[J]. Inorg Chem, 2015, 54(21):10440-10449.
doi: 10.1021/acs.inorgchem.5b01895 |
| [19] |
DUMON A, HUANG M, SHEN Y, et al. High Li ion conductivity in strontium doped Li7La3Zr2O12 garnet[J]. Solid State Ionics, 2013, 243:36-41.
doi: 10.1016/j.ssi.2013.04.016 |
| [20] |
YANG X, KONG D, CHEN Z, et al. Low-temperature fabrication for transparency Mg doping Li7La3Zr2O12 solid state electrolyte[J]. Journal of Materials Science:Materials in Electronics, 2017, 29(2):1523-1529.
doi: 10.1007/s10854-017-8062-4 |
| [21] |
DEVIANNAPOORANI C, SHANKAR L S, RAMAKUMAR S, et al. Investigation on lithium ion conductivity and structural stability of yttrium-substituted Li7La3Zr2O12[J]. Ionics, 2016, 22(8):1281-1289.
doi: 10.1007/s11581-016-1674-5 |
| [22] |
GU W, EZBIRI M, PRASADA RAO R, et al. Effects of penta-and trivalent dopants on structure and conductivity of Li7La3Zr2O12[J]. Solid State Ionics, 2015, 274:100-105.
doi: 10.1016/j.ssi.2015.03.019 |
| [23] |
LU Y, MENG X, ALONSO J A, et al. Effects of fluorine doping on structural and electrochemical properties of Li6.25Ga0.25La3Zr2O12 as electrolytes for solid-state lithium batteries[J]. ACS Appl Mater Interfaces, 2019, 11(2):2042-2049.
doi: 10.1021/acsami.8b17656 |
| [24] |
LIU C, WEN Z Y, RUI K. High ion conductivity in garnet-type F-doped Li7La3Zr2O12[J]. Journal of Inorganic Materials, 2015, 30(9):995-1001.
doi: 10.15541/jim20150163 |
| [25] |
LIU B, FU K, GONG Y, et al. Rapid thermal annealing of cathode-garnet interface toward high-temperature solid state batteries[J]. Nano Lett, 2017, 17(8):4917-4923.
doi: 10.1021/acs.nanolett.7b01934 |
| [26] | 金英敏, 李栋, 贾政刚, 等. 用于全固态锂电池的有机-无机复合电解质[J]. 原子与分子物理学报, 2020, 37(6):958-973. |
| [27] |
赵宁, 穆爽, 郭向欣. 石榴石型固态锂电池中的物理问题[J]. 物理学报, 2020, 69(22).Doi. org/10.7498/aps.69.20201191.
doi: org/10.7498/aps.69.20201191 |
| [28] | 何丹农, 张道明, 张芳. 有机无机复合固态电解质的制备方法及其产品和应用:中国,201811558680.3[P]. 2018-06-07. |
| [29] | ZHA W, CHEN F, YANG D, et al. High-performance Li6.4La3Zr1.4Ta0.6O12/Poly(ethylene oxide)/Succinonitrile composite electrolyte for solid-state lithium batteries[J]. Journal of Power So-urces, 2018, 397:87-94. |
| [30] |
ZHENG Y, YAO Y, OU J, et al. A review of composite solid-state electrolytes for lithium batteries:Fundamentals,key materials and advanced structures[J]. Chemical Society Reviews, 2020, 49(23):8790-8839.
doi: 10.1039/D0CS00305K |
| [31] |
ZHANG X, LIU T, ZHANG S, et al. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity,mechanical strength,and thermal stability of solid composite electrolytes[J]. J Am Chem Soc, 2017, 139(39):13779-13785.
doi: 10.1021/jacs.7b06364 |
| [32] |
ZHANG W, NIE J, LI F, et al. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane[J]. Nano Energy, 2018, 45:413-419.
doi: 10.1016/j.nanoen.2018.01.028 |
| [33] | 郭甜, 陈昱锜, 何泓材, 等. 基于有机-无机复合固态电解质膜的全固态锂电池制备与性能研究[J]. 电子元件与材料, 2019, 38(7):25-31. |
| [34] |
ZHANG X, XU B Q, LIN Y H, et al. Effects of Li6.75La3Zr1.75Ta0.25O12 on chemical and electrochemical properties of polyacrylonitrile-based solid electrolytes[J]. Solid State Ionics, 2018, 327:32-38.
doi: 10.1016/j.ssi.2018.10.023 |
| [35] |
TADANAGA K, TAKANO R, ICHINOSE T, et al. Low temperature synjournal of highly ion conductive Li7La3Zr2O12-Li3BO3 composit-es[J]. Electrochemistry Communications, 2013, 33:51-54.
doi: 10.1016/j.elecom.2013.04.004 |
| [36] |
PERSHINA S V, IL′INA E A, REZNITSKIKH O G. Phase Compo-sition,Density,and Ionic Conductivity of the Li7La3Zr2O12-Based Composites with LiPO3 Glass Addition[J]. Inorg Chem, 2017, 56(16):9880-9891.
doi: 10.1021/acs.inorgchem.7b01379 |
| [37] |
JANANI N, RAMAKUMAR S, KANNAN S, et al. Optimization of lithium content and sintering aid for maximized Li+ conductivit du-ctivity and density in Ta-doped Li7La3Zr2O12[J]. Journal of the American Ceramic Society, 2015, 98(7):2039-2046.
doi: 10.1111/jace.13578 |
| [38] | 吴学领, 苗艳丽, 王为, 等. 铌酸锂添加剂对石榴石型固体电解质Li6.75La3Zr1.75Sb0.25O12的致密度和离子电导率的影响[J]. 第31届全国化学与物理电源学术年会, 2015. |
| [39] |
TANG Y, ZHANG Q, LUO Z, et al. Effects of Li2O-Al2O3-SiO2 sy-stem glass on the microstructure and ionic conductivity of Li7La3Zr2O12 solid electrolyte[J]. Materials Letters, 2017, 193:251-254.
doi: 10.1016/j.matlet.2017.01.134 |
| [40] |
IL′INA E A, PERSHINA S V, ANTONOV B D, et al. The influence of the glass additive Li2O-B2O3-SiO2 on the phase composition, conductivity,and microstructure of the Li7La3Zr2O12[J]. Journal of Alloys and Compounds, 2018, 765:841-847.
doi: 10.1016/j.jallcom.2018.06.154 |
| [41] |
HUANG X, SHEN C, RUI K, et al. Influence of La2Zr2O7 additive on densification and Li+ conductivity for Ta-doped Li7La3Zr2O12 garnet[J]. Jom, 2016, 68(10):2593-2600.
doi: 10.1007/s11837-016-2065-0 |
| [42] |
KATO T, HAMANAKA T, YAMAMOTO K, et al. In-situ Li7La3Zr2O12 /LiCoO2 interface modification for advanced all-solid-state batt-ery[J]. Journal of Power Sources, 2014, 260:292-298.
doi: 10.1016/j.jpowsour.2014.02.102 |
| [43] |
WANG C, GONG Y, LIU B, et al. Conformal,nanoscale ZnO sur-face modification of garnet-based solid-state electrolyte for lithium metal anodes[J]. Nano Lett, 2017, 17(1):565-571.
doi: 10.1021/acs.nanolett.6b04695 |
| [44] |
XU H, LI Y, ZHOU A, et al. Li3N-modified garnet electrolyte for All-Solid-State lithium metal batteries operated at 40 degrees C[J]. Nano Lett, 2018, 18(11):7414-7418.
doi: 10.1021/acs.nanolett.8b03902 |
| [45] |
KOTOBUKI M, KOISHI M. High conductive Al-free Y-doped Li7La3Zr2O12 prepared by spark plasma sintering[J]. Journal of Alloys and Compounds, 2020, 826.Doi. org/10.1016/j.jallcom.2020.154213.
doi: org/10.1016/j.jallcom.2020.154213 |
| [46] |
CHEN R J, ZHANG Y B, LIU T, et al. Addressing the interface is-sues in all-solid-state bulk-type lithium ion battery via an all-co-mposite approach[J]. ACS Appl Mater Interfaces, 2017, 9(11):9654-9661.
doi: 10.1021/acsami.6b16304 |
| [47] |
ZHOU W, WANG S, LI Y, et al. Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte[J]. J Am Chem Soc, 2016, 138(30):9385-9388.
doi: 10.1021/jacs.6b05341 |
| [48] |
MIARA L J, ONG S P, MO Y, et al. Effect of Rb and Ta doping on the ionic conductivity and stability of the garnet Li7+2x-y(La3-xRbx)(Zr2-yTay)O12(0≤x≤0.375,0≤y≤1) superionic conductor:A first principles investigation[J]. Chemistry of Materials, 2013, 25(15):3048-3055.
doi: 10.1021/cm401232r |
| [49] |
SONG S, QIN X, RUAN Y, et al. Enhanced performance of solid-state lithium-air batteries with continuous 3D garnet network added composite polymer electrolyte[J]. Journal of Power Sources, 2020, 461.Doi. org/10.1016/j.jpowsour.2020.228146.
doi: org/10.1016/j.jpowsour.2020.228146 |
| [50] |
ZHANG W, WANG X, ZHANG Q, et al. Li7La3Zr2O12 ceramic nano-fiber-incorporated solid polymer electrolytes for flexible lithium batteries[J]. ACS Applied Energy Materials, 2020, 3(6):5238-5246.
doi: 10.1021/acsaem.0c00070 |
| [51] | WANG C, FU K, KAMMAMPATA S P, et al. Garnet-type solid-st-ate electrolytes:Materials,interfaces,and batteries[J]. ChemRev, 2020, 120(10):4257-4300. |
| [52] |
MCOWEN D W, XU S, GONG Y, et al. 3D-printing electrolytes for solid-state batteries[J]. AdvMater, 2018, 30(18).Doi. org/10.1002/adma.201707132.
doi: org/10.1002/adma.201707132 |
| [1] | KANG Le, JING Maoxiang, LI Donghong, HU Xinyu, JIA Chunyan. Study on preparation and electrochemical performance of lithium aluminate nanorods modified solid electrolyte [J]. Inorganic Chemicals Industry, 2023, 55(8): 65-70. |
| [2] | YU Hui, WANG Yubin, LIAO Zhejun, YANG Yunguang. Overview of recycling and utilization process of waste ternary lithium-ion power batteries [J]. Inorganic Chemicals Industry, 2023, 55(7): 32-37. |
| [3] | ZHU Zhihong, ZHU Yongfang. Study on preparation and properties of silicon doped lithium manganate by self-propagating combustion [J]. Inorganic Chemicals Industry, 2023, 55(5): 66-70. |
| [4] | LIU Jinhang,YANG Zhipeng,CHEN Xiudong,LUO Yuxuan,YU Langhua,WANG Yawei,ZHAN Changchao,CAO Xiaohua. Preparation of new porous carbon and its lithium storage performance [J]. Inorganic Chemicals Industry, 2022, 54(9): 85-89. |
| [5] | KUANG Xinliang,LIU Chuixiang,XIONG Peng. Industry analysis and market prospect of lithium ion battery [J]. Inorganic Chemicals Industry, 2022, 54(8): 12-19. |
| [6] | ZHOU Lei,YUAN Yongshun,LI Lu,LIU Bingguang,LI Jiansheng. Research progress on preparation and application of lithium ion sieve from spent lithium ion batteries [J]. Inorganic Chemicals Industry, 2022, 54(8): 33-39. |
| [7] | Sun Xinhua,Hou Lei,Qin Kai. Market analysis of lithium hexafluorophosphate liquid electrolytes in lithium-ion battery [J]. Inorganic Chemicals Industry, 2021, 53(3): 7-11. |
| [8] | HU Yue,XU Lele,ZHOU Yanfang,XU Zhe,TIAN Shoushuai,LIAO Fan,YANG Xiaojun. Research progress on alkaline substance on surface of nickel-rich ternary cathode materials [J]. Inorganic Chemicals Industry, 2021, 53(12): 74-79. |
| [9] | Liu Yang,Cai Zongying,Cao Weigang,Liu Yuzhao. Research progress on lithium sodium titanate for lithium ion batteries [J]. Inorganic Chemicals Industry, 2021, 53(10): 36-40. |
| [10] | Wang Kuangbin,Xu Shengxia,Wang Yongqin. Research on purification process of lithium difluorosulfimide optimized by response surface method [J]. Inorganic Chemicals Industry, 2020, 52(9): 62-65. |
| [11] | Wang Jiatai,Zhao Duan,Ma Lianhua,Zhang Caihong. Research progress of LiFePO4 cathode materials for Li-ion battery [J]. Inorganic Chemicals Industry, 2020, 52(4): 18-22. |
| [12] | Luo Chengguo,Xiao Jun,Fan Guangxin. Current situation and development of precursors for LiMn2O4 cathode used in lithium ion batteries [J]. Inorganic Chemicals Industry, 2020, 52(1): 26-29. |
| [13] | Sun Peiliang,Chen Shijuan,Yuan Li. Preparation and electrochemical properties of lithium difluorophosphate [J]. Inorganic Chemicals Industry, 2019, 51(5): 45-48. |
| [14] | LIANG Da-Yu. Effect of PVDF binder content on rheological properties of cathode slurry for lithium ion batteries [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(8): 77-. |
| [15] | BIAN Du-Cheng, LIU Shu-Lin, TIAN Yuan. Reusing of spent LiFePO4 cathode materials by solid phase lithium refilling method and electrochemical performance there of [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 71-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||