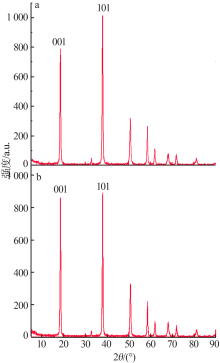
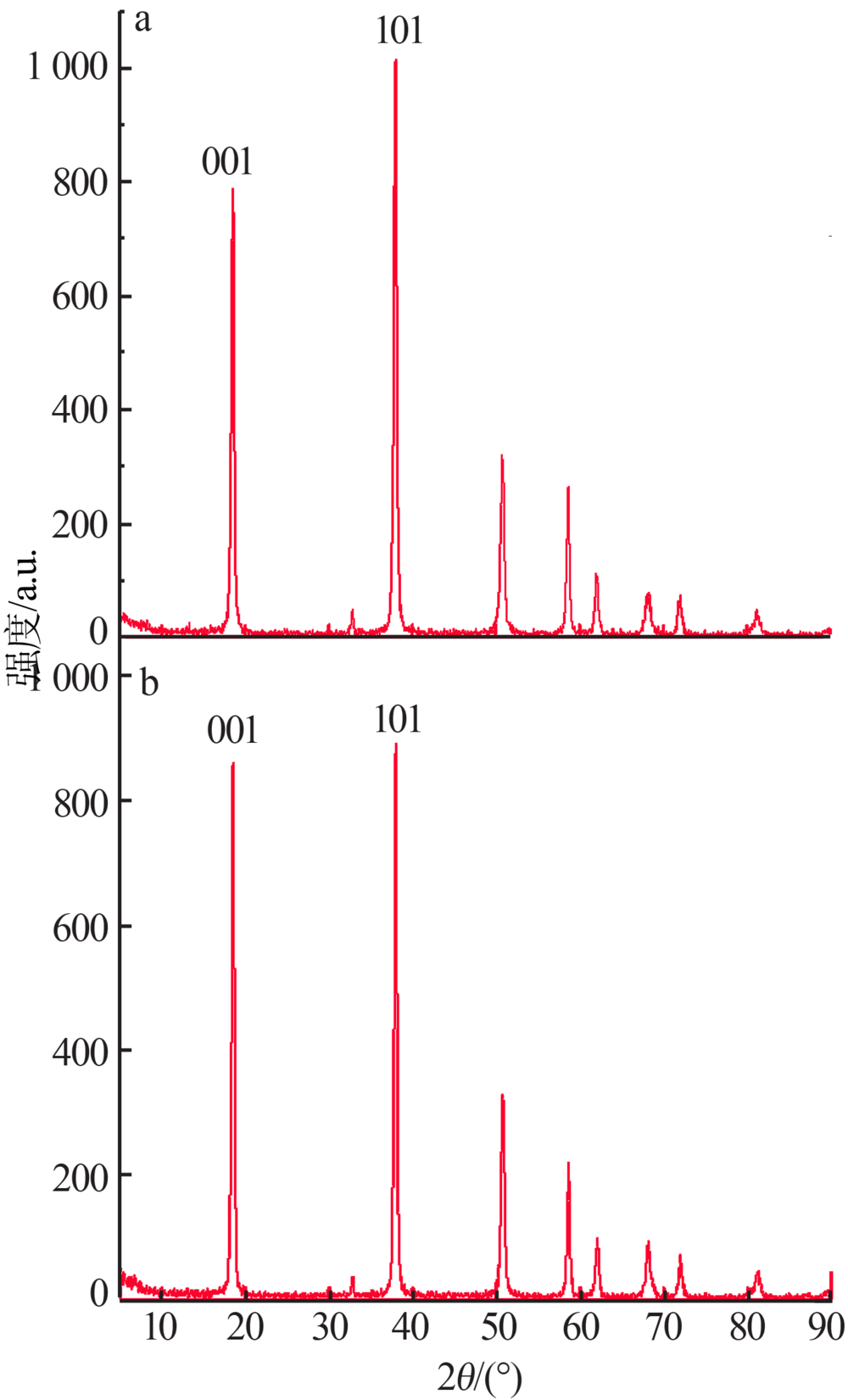
Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (5): 23-27.
• Research & Development • Previous Articles Next Articles
Application of TRIZ theory in preparation of magnesium hydroxide by light burning ammonia
Fan Tianbo1,Jiang Yu1,Chen Si1,You Gang1,Ding Ke1,Sun Xiaojun1,Liu Luping1,Li Li2,Liu Yunyi1( ),Hu Kaizhou1,Ma Junru1,Guo Xiyao1
),Hu Kaizhou1,Ma Junru1,Guo Xiyao1
- 1. College of Chemical Engineering,Shenyang University of Chemical Technology,Liaoning Provincial Key Laboratory of Chemical Application Technology,Shenyang 110142,China
2. School of Computer Science and Technology,Shenyang University of Chemical Technology