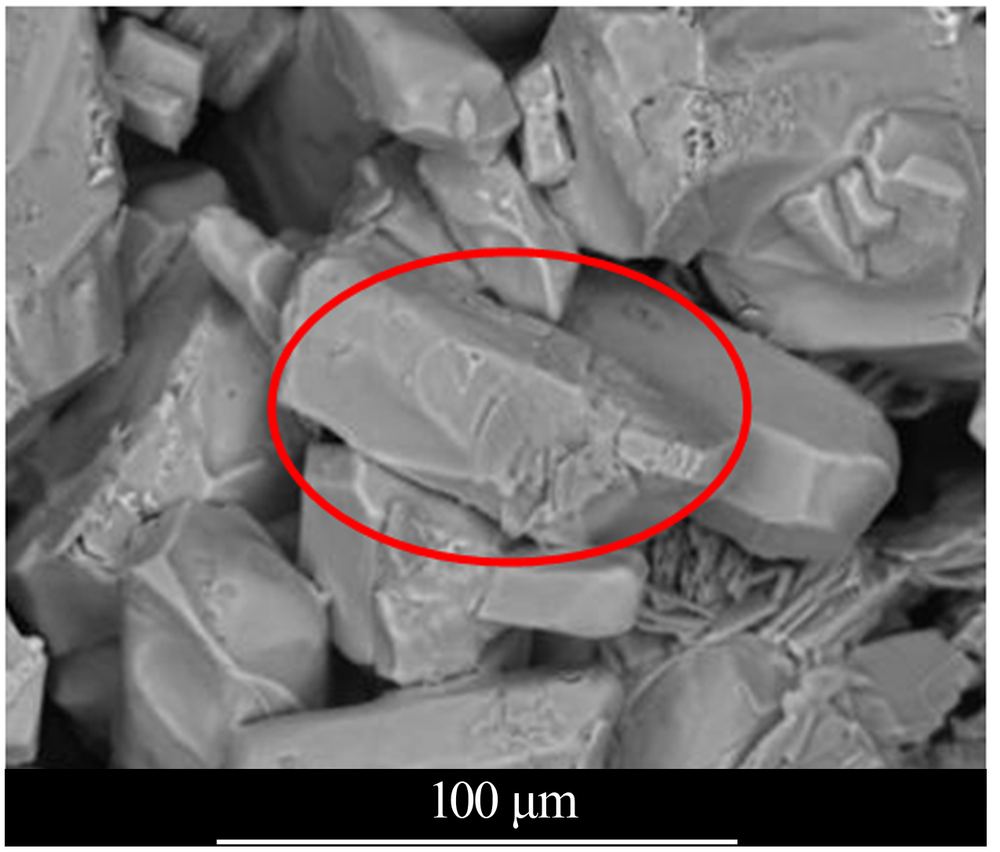
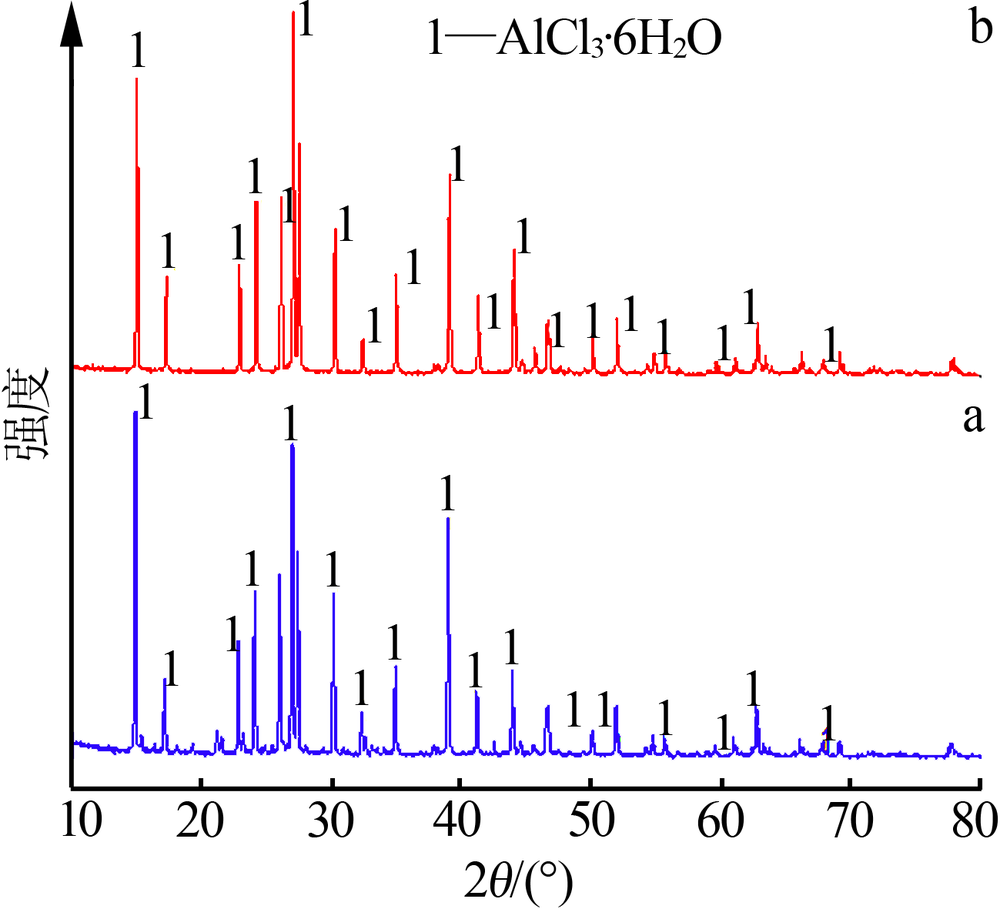
| [1] |
吴兴科 . 无水三氯化铝及结晶三氯化铝的生产工艺和应用[J]. 精细化工, 1988(3):31-34.
|
| [2] |
年志高, 李剑萍 . 三氯化铝应用性能探讨[J]. 无机盐工业, 1985,17(4):40-42.
|
| [3] |
Guo Y, Lv H, Yang X , et al. AlCl3·6H2O recovery from the acid leaching liquor of coal gangue by using concentrated hydrochloric inpouring[J]. Separation & Purification Technology, 2015,151:177-183.
|
| [4] |
魏存弟, 杨殿范, 张东丽 , 等. 循环流化床粉煤灰制备结晶氯化铝的方法:中国,101054192A[P].2007-10-17.
|
| [5] |
郭昭华, 王永旺, 张小东 , 等. 一种制备高纯结晶氯化铝的方法:中国,103979590A[P].2014-08-13.
|
| [6] |
Maysilles J H, Traut D E, Sawyer D L . Aluminum chloride hexahydrate crystallization by HCl gas sparging[M]. US:Bureau of Mine, 1982.
|
| [7] |
刘立新, 杜卫民, 朱晓燕 , 等. MoO3纳米材料的形貌控制合成及其可见光催化性能研究[J]. 化工新型材料, 2015(6):184-187.
|
| [8] |
王丽丽, 李嘉荣, 唐定中 . 矿化剂氧化铝的形貌对二氧化硅基陶瓷型芯性能的影响[J]. 航空材料学报, 2015,35(1):8-12.
doi: 10.11868/j.issn.1005-5053.2015.1.002
|
| [9] |
徐涛, 兰海平, 杨超 , 等. 粉磨酸浸-氯化氢通气结晶法提取粉煤灰中氧化铝[J]. 无机盐工业, 2018,50(1):57-61.
|
| [10] |
Wang P, Li C, Gong H , et al. Morphology control and growth mech-anism of magnesium hydroxide nanoparticles via a simple wet pre-cipitation method[J]. Ceramics International, 2011,37(8):3365-3370.
doi: 10.1016/j.ceramint.2011.05.138
|
| [11] |
Chojjati H, Rohani S . Cooling and seeding effect on supersaturation and final crystal size distribution(CSD) of ammonium sulphate in a batch crystallizer[J]. Chemical Engineering & Processing Process Intensification, 2005,44(9):949-957.
|
| [12] |
徐阳, 孙志丹, 陈晓浪 , 等. 结晶温度对左旋聚乳酸的晶体改性和晶体形貌的影响[J]. 功能材料, 2012,43(16):2138-2141.
|
| [13] |
王锦华, 杨新亚, 王守兴 , 等. Na4Ca(SO4)3复盐高温合成及温度对晶体形貌的影响[J]. 无机化学学报, 2007,23(5):791-794.
|
| [14] |
闫平科, 田海山, 高玉娟 , 等. 反应物浓度对三水碳酸镁晶体生长形貌的影响研究[J]. 硅酸盐通报, 2013,32(12):2568-2571.
|
| [15] |
张建华, 曹付明, 张宏建 , 等. 初始过饱和度对谷氨酸结晶的影响研究[J]. 高校化学工程学报, 2013(1):43-49.
|
 ),Cheng Fangqin
),Cheng Fangqin