Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (10): 110-116.doi: 10.11962/1006-4990.2019-0609
• Industrial Techniques • Previous Articles Next Articles
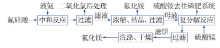
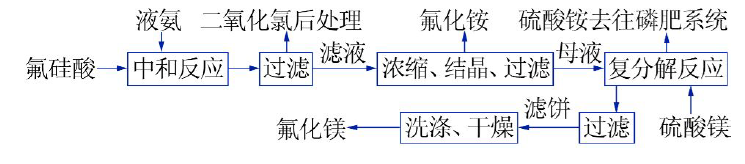
Study on process technology of preparing NH4F and co-producing MgF2 with high impurity fluosilicic acid
Huang Zhong( ),Yu Shuangqiang,Gao Kaiyuan,He Jun,Huang He,Zhang Xianfeng,Chen Xizhen
),Yu Shuangqiang,Gao Kaiyuan,He Jun,Huang He,Zhang Xianfeng,Chen Xizhen
- Hubei Xiangyun(Group) Chemical Co.,Ltd.,Wuxue 435400,China
-
Received:2020-04-24Online:2020-10-10Published:2020-11-24
CLC Number:
Cite this article
Huang Zhong,Yu Shuangqiang,Gao Kaiyuan,He Jun,Huang He,Zhang Xianfeng,Chen Xizhen. Study on process technology of preparing NH4F and co-producing MgF2 with high impurity fluosilicic acid[J]. Inorganic Chemicals Industry, 2020, 52(10): 110-116.
share this article
"
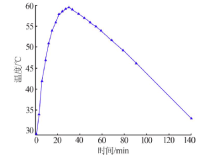
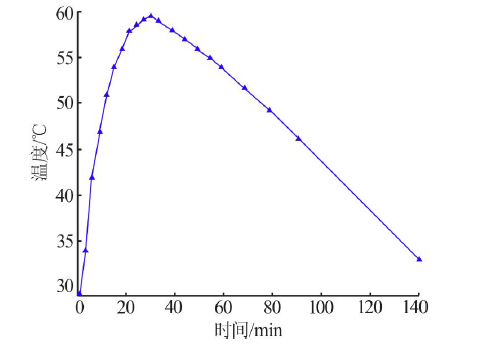
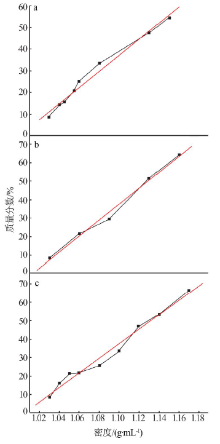
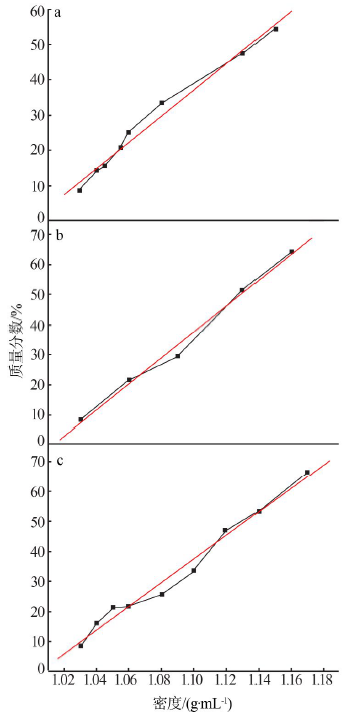
| 序号 | 反应 温度/℃ | 过滤速度/ (mL·min-1) | 过滤速度平均值/(mL·min-1) | 滤液中 w(F)/% | 滤液中w(F)平均值/% | 硅胶中 w(F)/% | 硅胶中w(F) 平均值/% | 硅胶含 水量/% | 硅胶含水量 平均值/% |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 5.8 | 5.9 | 77 | 74 | 23 | 26 | 68 | 68 |
| 2 | 40 | 5.8 | 73 | 27 | 66 | ||||
| 3 | 40 | 5.9 | 72 | 28 | 68 | ||||
| 4 | 60 | 7.2 | 7.4 | 78 | 79 | 22 | 22 | 66 | 66 |
| 5 | 60 | 7.6 | 78 | 22 | 64 | ||||
| 6 | 60 | 7.5 | 79 | 21 | 67 | ||||
| 7 | 70 | 9.1 | 9.8 | 81 | 80 | 19 | 20 | 65 | 63 |
| 8 | 70 | 9.8 | 79 | 21 | 62 | ||||
| 9 | 70 | 10.5 | 81 | 19 | 63 | ||||
| 10 | 80 | 12.9 | 14.0 | 85 | 85 | 15 | 15 | 62 | 61 |
| 11 | 80 | 18.2 | 86 | 14 | 59 | ||||
| 12 | 80 | 11.0 | 84 | 16 | 62 |
"
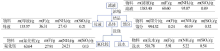
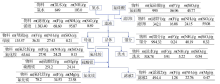
| 序号 | 洗涤方式 | 硅胶 w(F)/% | 一次洗涤 w(F)/% | 一次洗涤 率/% | 二次洗涤 w(F)/% | 二次洗涤 率/% | 三次洗涤 w(F)/% | 三次洗涤 率/% | 四次洗涤 w(F)/% | 四次洗涤 率/% | 洗涤率 合计/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 再浆 | 3.94 | 1.34 | 68.02 | 0.34 | 17.26 | 0.08 | 4.06 | 0.02 | 1.01 | 90.35 |
| 置换 | 3.94 | 1.30 | 65.99 | 0.19 | 9.64 | 0.07 | 3.55 | 0.04 | 2.03 | 81.21 | |
| 2 | 再浆 | 3.91 | 1.20 | 61.38 | 0.33 | 16.88 | 0.08 | 4.09 | 0.02 | 1.02 | 83.37 |
| 置换 | 3.91 | 1.33 | 68.03 | 0.13 | 6.65 | 0.07 | 3.58 | 0.04 | 2.05 | 80.31 | |
| 3 | 再浆 | 3.91 | 1.30 | 66.50 | 0.31 | 15.86 | 0.08 | 4.09 | 0.03 | 1.53 | 87.98 |
| 置换 | 3.91 | 1.24 | 63.43 | 0.17 | 8.70 | 0.07 | 3.58 | 0.04 | 2.05 | 77.76 | |
| 4 | 再浆 | 3.90 | 1.24 | 63.59 | 0.26 | 13.33 | 0.09 | 4.62 | 0.05 | 2.56 | 84.10 |
| 置换 | 3.90 | 1.17 | 60.00 | 0.12 | 6.15 | 0.06 | 3.08 | 0.05 | 2.56 | 71.79 | |
| 平均值 | 再浆 | 64.87 | 15.83 | 4.22 | 1.53 | 86.45 | |||||
| 置换 | 64.36 | 7.79 | 3.45 | 2.17 | 77.77 |
| [1] | 薛河南, 明大增, 李志祥, 等. 磷肥副产氟硅酸制白炭黑技术[J]. 无机盐工业, 2007,39(5):8-9. |
| [2] | 李洁, 张晓霞, 张梅. 用磷肥副产氟硅酸制氟化铵和白炭黑工艺研究[J]. 无机盐工业, 2009,41(1):55-56. |
| [3] | 叶文龙, 黄少清, 丛海辉. 我国氟化铵、氟化氢铵生产、技术现状及发展趋势[J]. 化工生产与技术, 2011,11(6):12-14,24. |
| [4] | 肖冠斌, 丁一刚, 邓伏礼, 等. 湿法磷酸液相氟制备氟化铵的工艺研究[J]. 化工矿物与加工, 2015(11):14-17. |
| [5] | 孔小雁, 黄忠, 余莹, 等. 磷矿脱镁废液制备氟化镁工艺研究[J]. 无机盐工业, 2019,51(3):57-58,62. |
| [6] | 黄忠, 孔小雁, 余莹, 等. 利用磷矿脱镁废液制备MF-1级氟化镁工艺技术研究[J]. 化工矿物与加工, 2019(6):48-51. |
| [7] | 李天祥. 氟化铵结晶过程的研究[D]. 贵阳:贵州大学, 2010. |
| [1] | Sui Yanfeng,Liu Songlin,Qin Hong. Experimental study on preparation of ammonium fluosilicate from fluorinated silica slag dissolved by ammonium fluoride [J]. Inorganic Chemicals Industry, 2020, 52(1): 76-78. |
| [2] | Kong Xiaoyan,Huang Zhong,Yu Ying,Ju Li,Liang Lei,He Jun. Study on the preparation of magnesium fluoride from demagging liquor of phosphate ore [J]. Inorganic Chemicals Industry, 2019, 51(3): 57-58. |
| [3] | Zhao Yuxiang1,2,3,Huang Peijin1,Ma Zhenying4,Li Xiao1,2,3,Li Bo1,2,Zou Xingwu1,2,Wang Shuxuan1,2. Preparation of high purity magnesium fluoride by magnesium sulfate composite refining and fluorination [J]. Inorganic Chemicals Industry, 2019, 51(12): 23-25. |
| [4] | CUI Xiang-Mei, LIU Hai-Ning, WEN Xian-Ming. Brine entrainment rate and material balance of Yiliping salt brine evaporation [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 33-. |
| [5] | WANG Yong-Mei, LI Chun-Li, LIU Liang-Mei. Phase diagram analysis and calculation of salt pan process of Chaerhan salt lake [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 26-. |
| [6] | FU Jing-Chun. Study on preparation of high purity spherical barium carbonate particles [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(10): 50-. |
| [7] | CHEN Wen-Xing, TIAN Juan, ZHOU Chang-Ping. Preparation technology of potassium sulfate with insoluble potassium contained shale [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(7): 42-. |
| [8] | ZHANG Xiao-Xia. Study on new production technology of battery grade lithium fluoride [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(10): 55-. |
| [9] | LUO Fan, CHEN Jie, JIN Xin, WU Wei, JIAO Ke-Xin. Analysis on online mathematical model for submerged arc furnace power-saving control [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(3): 38-. |
| [10] | YU Zheng-Xing, MING Da-Zeng, LI Zhi-Xiang, NIU Yong-Sheng, YIN Li-Ting, YANG Yu-Jing. Synthesis process of ammonium fluoride [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(6): 9-. |
| [11] | XU Jin-Yao, MING Da-Zeng, LI Zhi-Xiang, HE Hong-Liang. Preparation methods of magnesium fluoride [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(9): 8-. |
| [12] | WU Hai-Feng, LIU Hai-Xia, HOU Li-Fang. Experimental research on product quality improvement of ammonium fluoride [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(4): 45-. |
| [13] | XU Chun-Xiao, LIU Xiao-Hong, KE Chun-Lan, ZHANG Xiao-Ming. Preparation technology of high purity barium fluoride from ammonium salt [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(4): 47-. |
| [14] | Li Jie;Zhang Xiaoxia;Zhang Mei. Manufacturing technology of ammonium fluoride and white carbon black from fluosilicic acid by-produced in phosphate fertilizer industry [J]. INORGANICCHEMICALSINDUSTRY, 2009, 0(1): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|