Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (11): 33-36.doi: 10.11962/1006-4990.2019-0674
• Research & Development • Previous Articles Next Articles
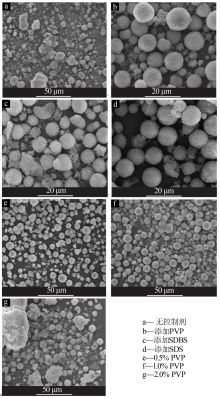
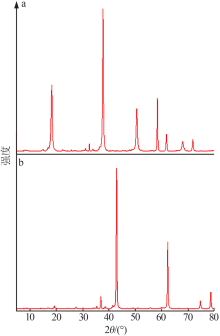
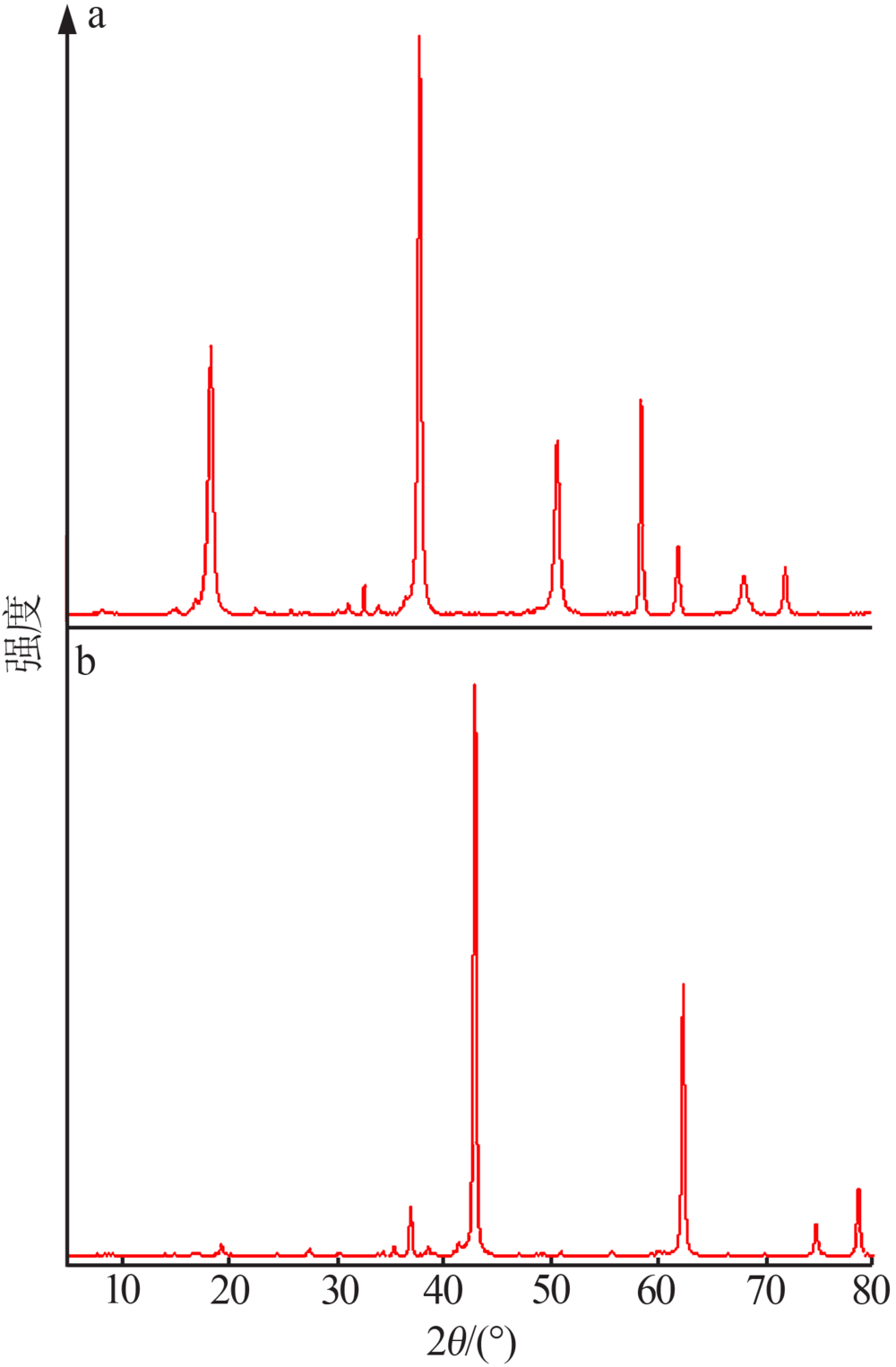
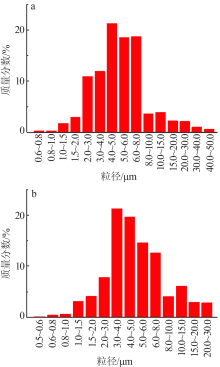
Study on controllable synthesis of spherical magnesium oxide
Zhu Huayang1,2,3,Zhong Jianchu1,2,3( ),Yu Benru1,Liu Jianxing1,Luo Xinhu1
),Yu Benru1,Liu Jianxing1,Luo Xinhu1
- 1. School of Chemical Engineering,Dalian University of Technology,Dalian 116024,China,2.Engineering Laboratory of Boric and Magnesic Functional Material Preparative and Applied Technology in Liaoning Province
2. Engineering Laboratory of Boric and Magnesic Functional Material Preparative and Applied Technology in Liaoning Province
3. Liaoning Provincial Chemical Process and Advanced Materials Research Center on B & Mg Natural Resources