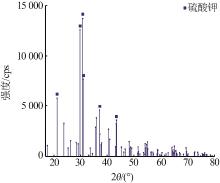
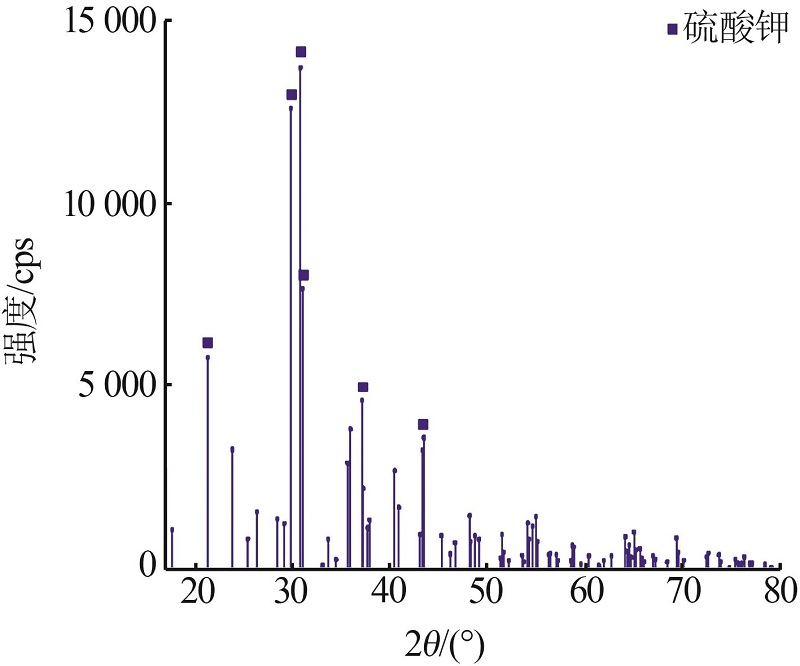
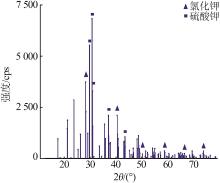
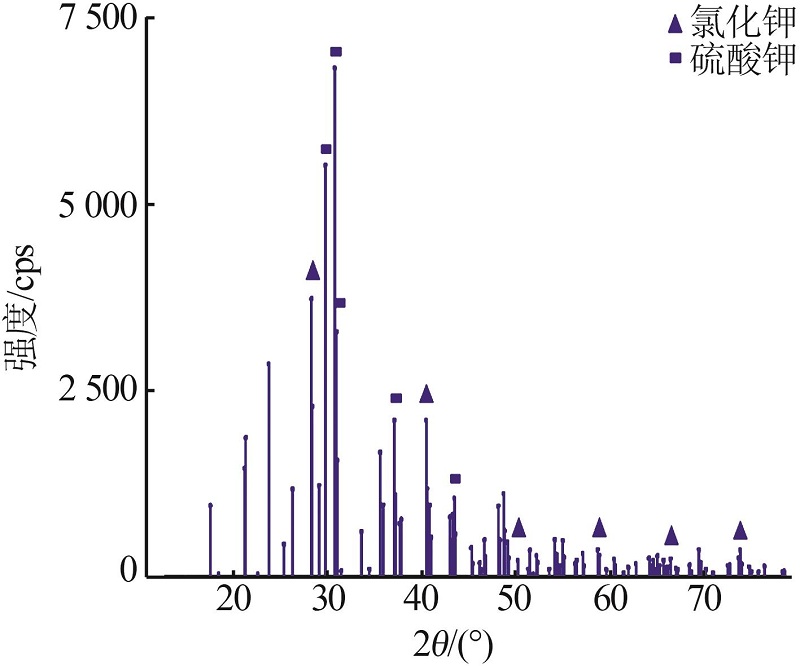
| 1 |
杨卉芃,曹飞.世界钾资源研究系列之一:资源概况及供需分析[J].矿产保护与利用,2015(1):75-78.
|
|
YANG Huipeng, CAO Fei.Series study on potassium resources in world:General situation and analysis of supply and demand[J].Conservation and Utilization of Mineral Resources,2015(1):75-78.
|
| 2 |
熊增华,王石军.中国钾资源开发利用技术及产业发展综述[J].矿产保护与利用,2020,40(6):1-7.
|
|
XIONG Zenghua, WANG Shijun.Overview of potassium resources exploitation & utilization technology and potash industry development[J].Conservation and Utilization of Mineral Resources,2020,40(6):1-7.
|
| 3 |
王鑫,李恩泽,程芳琴.国内外钾资源及钾肥生产现状[J].广州化工,2018,46(14):9-10.
|
|
WANG Xin, LI Enze, CHENG Fangqin.Present situation of domestic and abroad potassium resources and potash production[J].Guangzhou Chemical Industry,2018,46(14):9-10.
|
| 4 |
郭占成,彭翠,张福利,等.利用钢铁企业烧结电除尘灰生产氯化钾的方法:中国,200810101269.3[P].2010-06-02.
|
| 5 |
闫新平,蒋宝华,刘竹焕.综合处理工业废弃物的方法:中国,201110040653.9[P].2013-01-30.
|
| 6 |
王笑,吕扬,仪垂杰,等.烧结机头电除尘灰的资源化应用现状[J].科技创新与应用,2022,12(10):50-53,58.
|
|
WANG Xiao, Yang LÜ, YI Chuijie,et al.Present situation of resource application of electrostatic precipitator dust in sintering machine head[J].Technology Innovation and Application,2022,12(10):50-53,58.
|
| 7 |
郭占成,詹光.一种利用水泥窑灰生产氯化钾及联产碳酸钙的方法:中国,201410074515.6[P].2016-05-11.
|
| 8 |
张向南.关于利用水泥窑灰生产钾肥的分析[J].化工管理,2016(29):271.
|
| 9 |
周建国,张曙光,李萍,等.城市生活垃圾焚烧飞灰水洗脱氯实验研究[J].天津城建大学学报,2015,21(6):417-422.
|
|
ZHOU Jianguo, ZHANG Shuguang, LI Ping,et al.Experimental study of removal of soluble chlorides by water-washing pretreatment of municipal solid waste incineration fly ash[J].Journal of Tianjin Chengjian University,2015,21(6):417-422.
|
| 10 |
郭煜诚.MSWI飞灰脱盐及固化重金属性能研究[D].徐州:中国矿业大学,2021.
|
|
GUO Yucheng.Research on granulation desalination of MSWI (municipal solid waste incineration) fly ash and solidification of heavy metals[D].Xuzhou:China University of Mining and Technology,2021.
|
| 11 |
常威.生活垃圾焚烧飞灰的水洗及资源化研究[D].杭州:浙江大学,2016.
|
|
CHANG Wei.Study on the washing process and recycling of MSWI fly ash[D].Hangzhou:Zhejiang University,2016.
|
| 12 |
张晓樵.生活垃圾焚烧飞灰毒性浸出规律及水洗预处理废水资源化处理探索[D].上海:上海大学,2015.
|
|
ZHANG Xiaoqiao.Leaching characteristics of heavy metals in MSWI fly ash and resource recovery from wastewater during pre-treatment process[D].Shanghai:Shanghai University,2015.
|
| 13 |
王月香,邵兰燕,徐天男,等.垃圾焚烧飞灰中氯元素存在形态及深度脱氯的研究[J].无机盐工业,2021,53(5):78-83.
|
|
WANG Yuexiang, SHAO Lanyan, XU Tiannan,et al.Study on existing speciation and deep dechlorination of chlorine in waste incineration fly ash[J].Inorganic Chemicals Industry,2021,53(5):78-83.
|
| 14 |
常威,刘宏辉,蒋旭光.垃圾焚烧飞灰水洗脱氯及重金属浸出特性研究[J].无机盐工业,2022,54(3):113-118.
|
|
CHANG Wei, LIU Honghui, JIANG Xuguang.Study on dechlorination and heavy metal leaching characteristics of MSWI fly ash during water-washing[J].Inorganic Chemicals Industry,2022,54(3):113-118.
|
| 15 |
潘明亮,戚丽娜,王军堂,等.利用烧结法熟料窑窑灰生产氯化钾的方法:中国,200710015645.2[P].2007-11-07.
|
| 16 |
梁保民.水盐体系相图原理及运用[M].北京:轻工业出版社,1986.
|
| 17 |
牛自得,程芳琴.水盐体系相图及其应用[M].天津:天津大学出版社,2002.
|
| 18 |
中国科学院青海盐湖研究所分析室.卤水和盐的分析方法[M].2版.北京:科学出版社,1988.
|
 ), WANG Min1,2,3(
), WANG Min1,2,3( ), GE Haiwen1,2,3, QIAO Youmin4, QIAO Ziyang4
), GE Haiwen1,2,3, QIAO Youmin4, QIAO Ziyang4