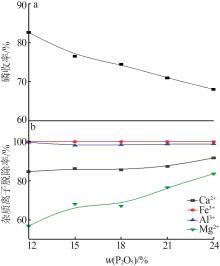
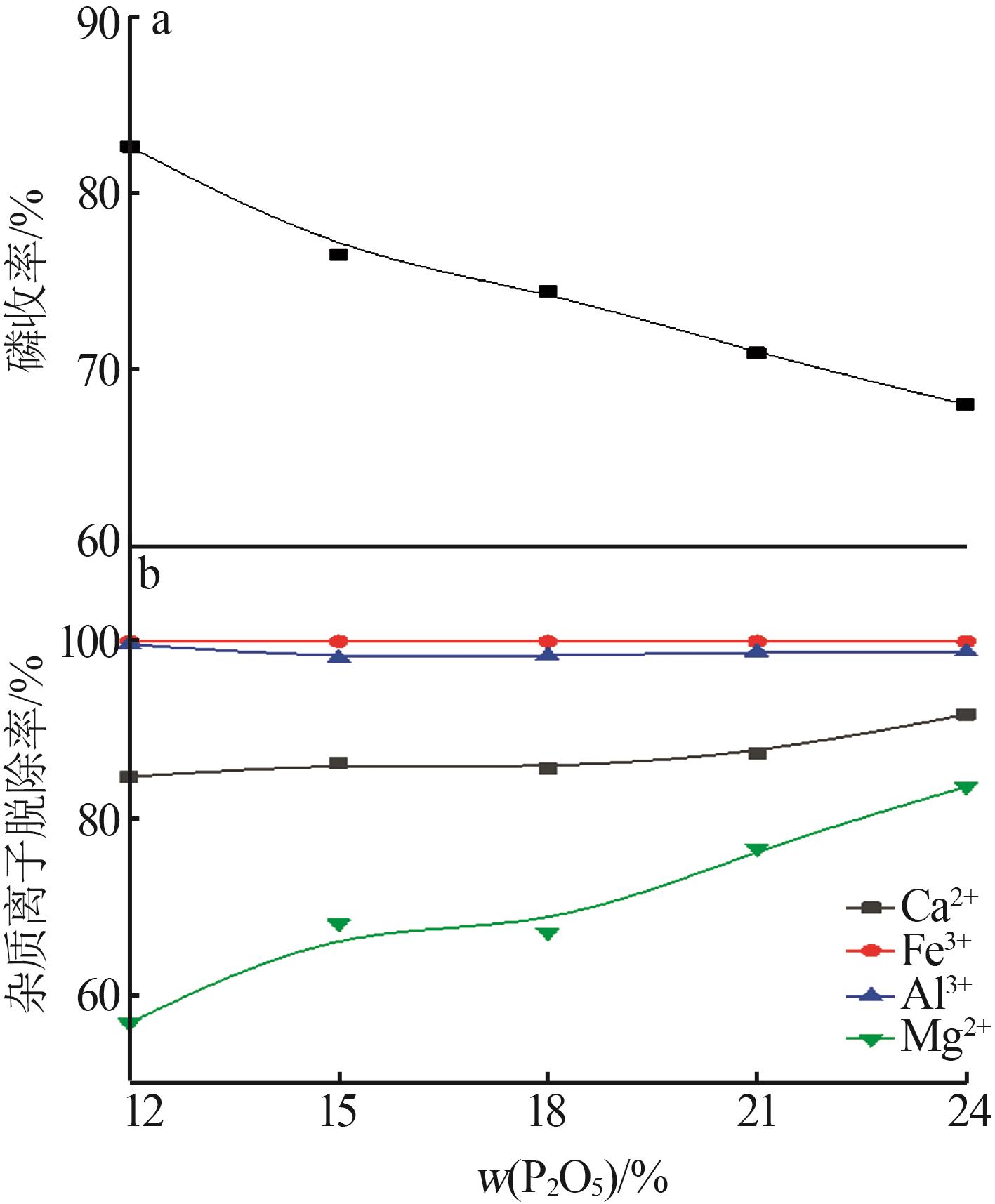
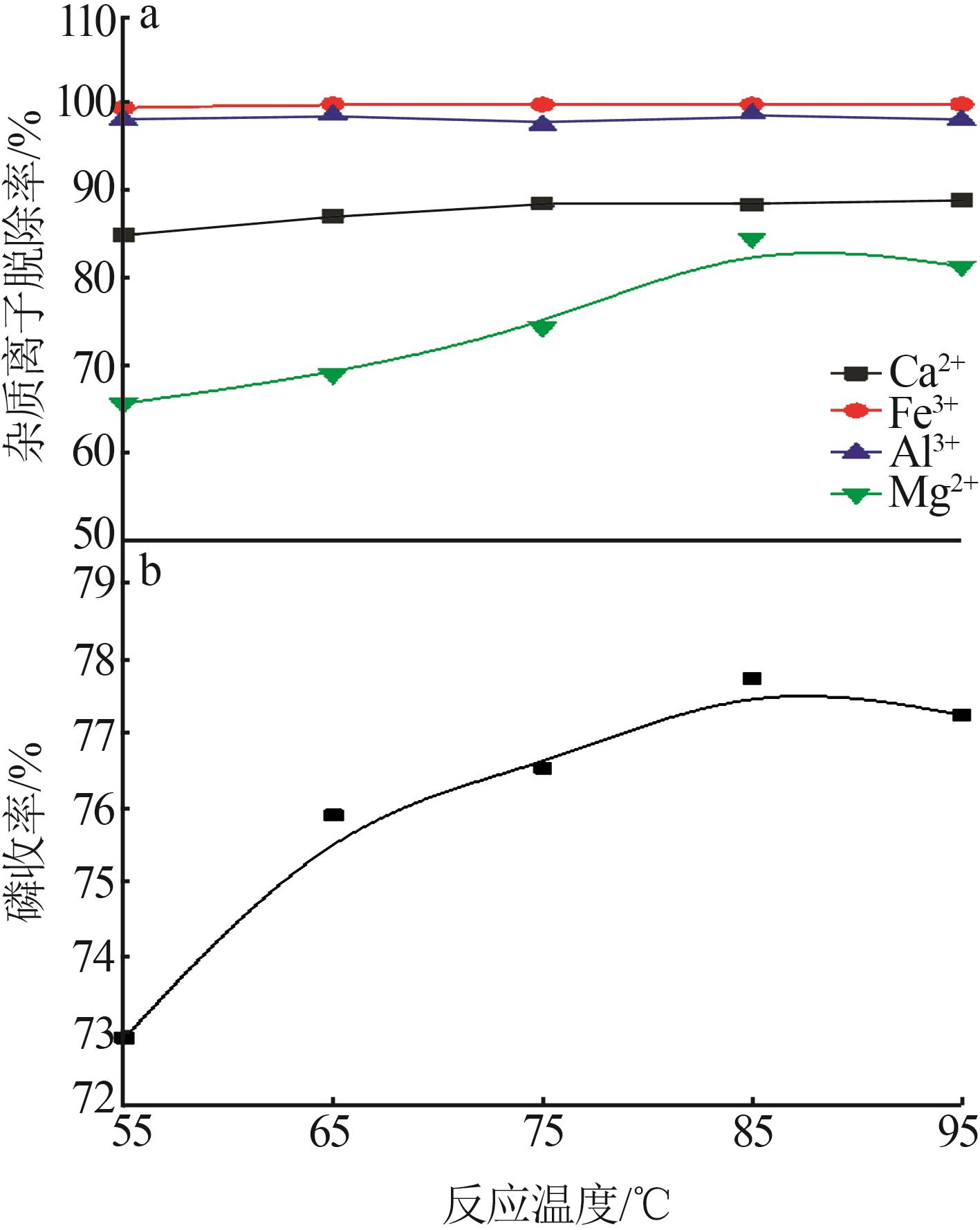
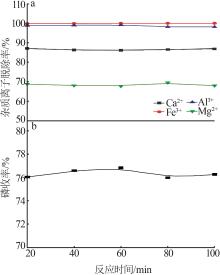
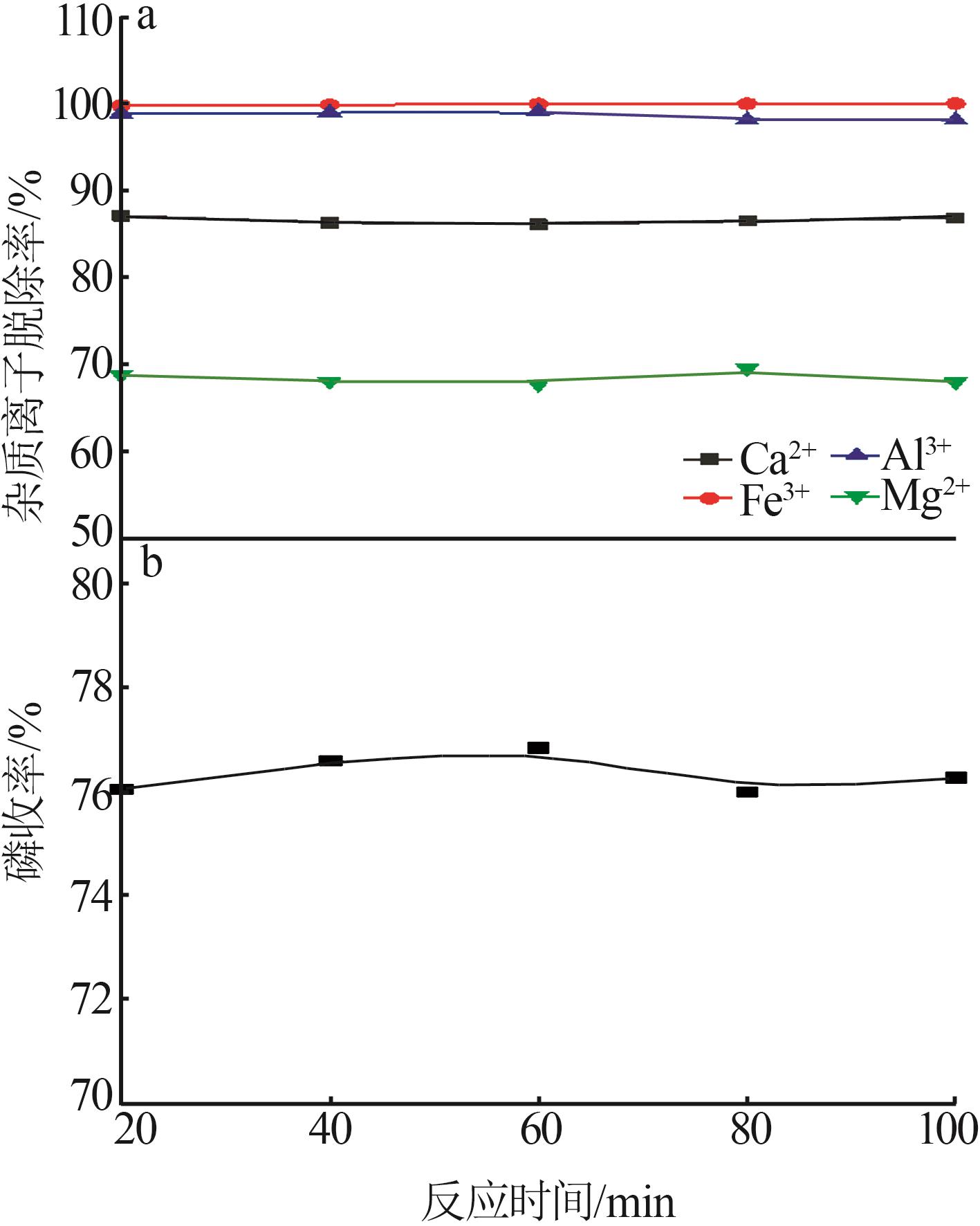
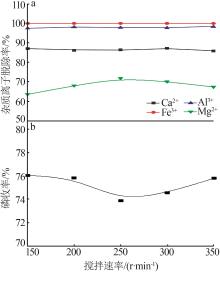
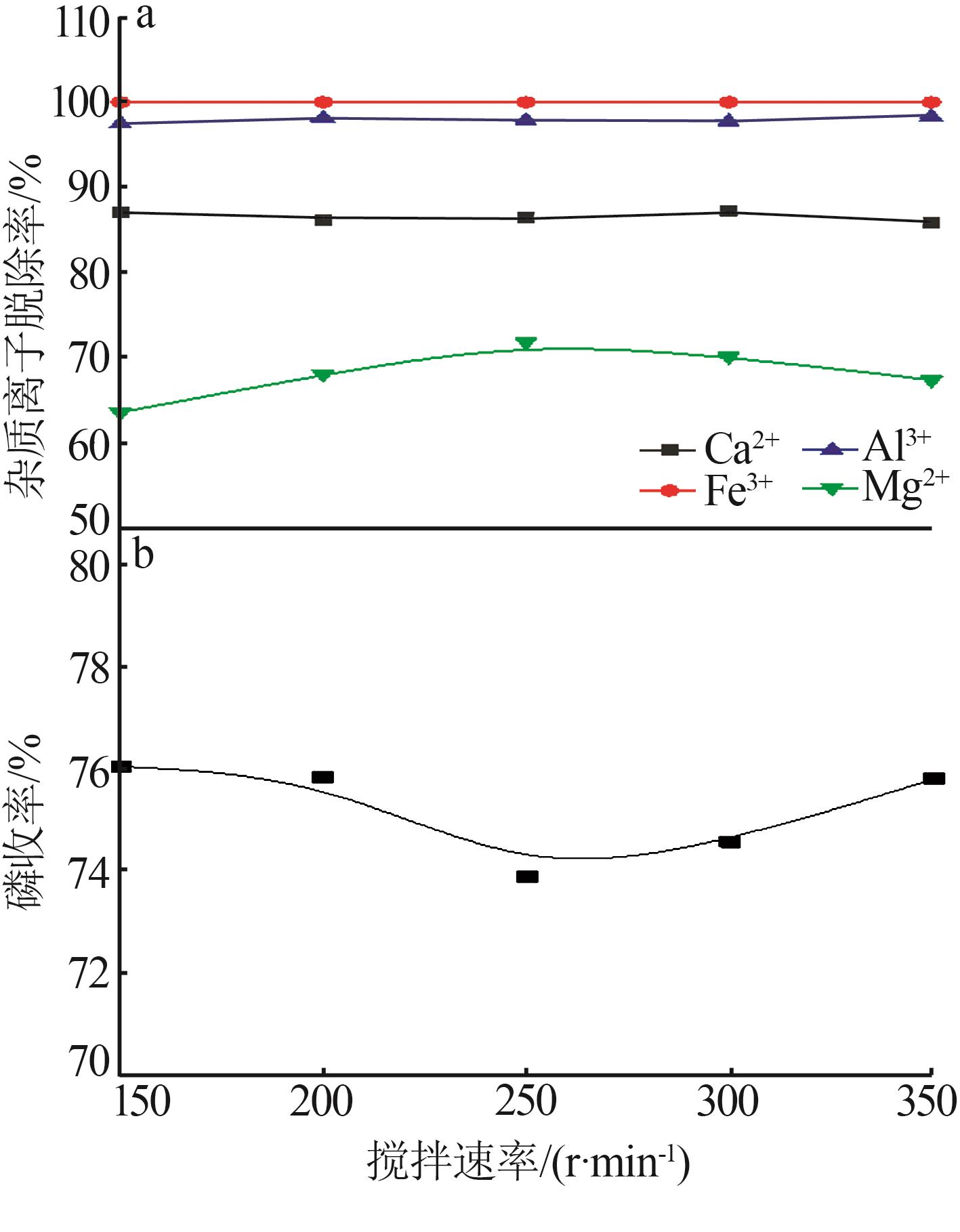
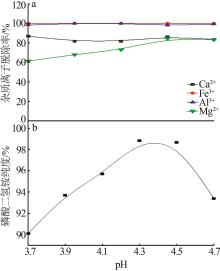
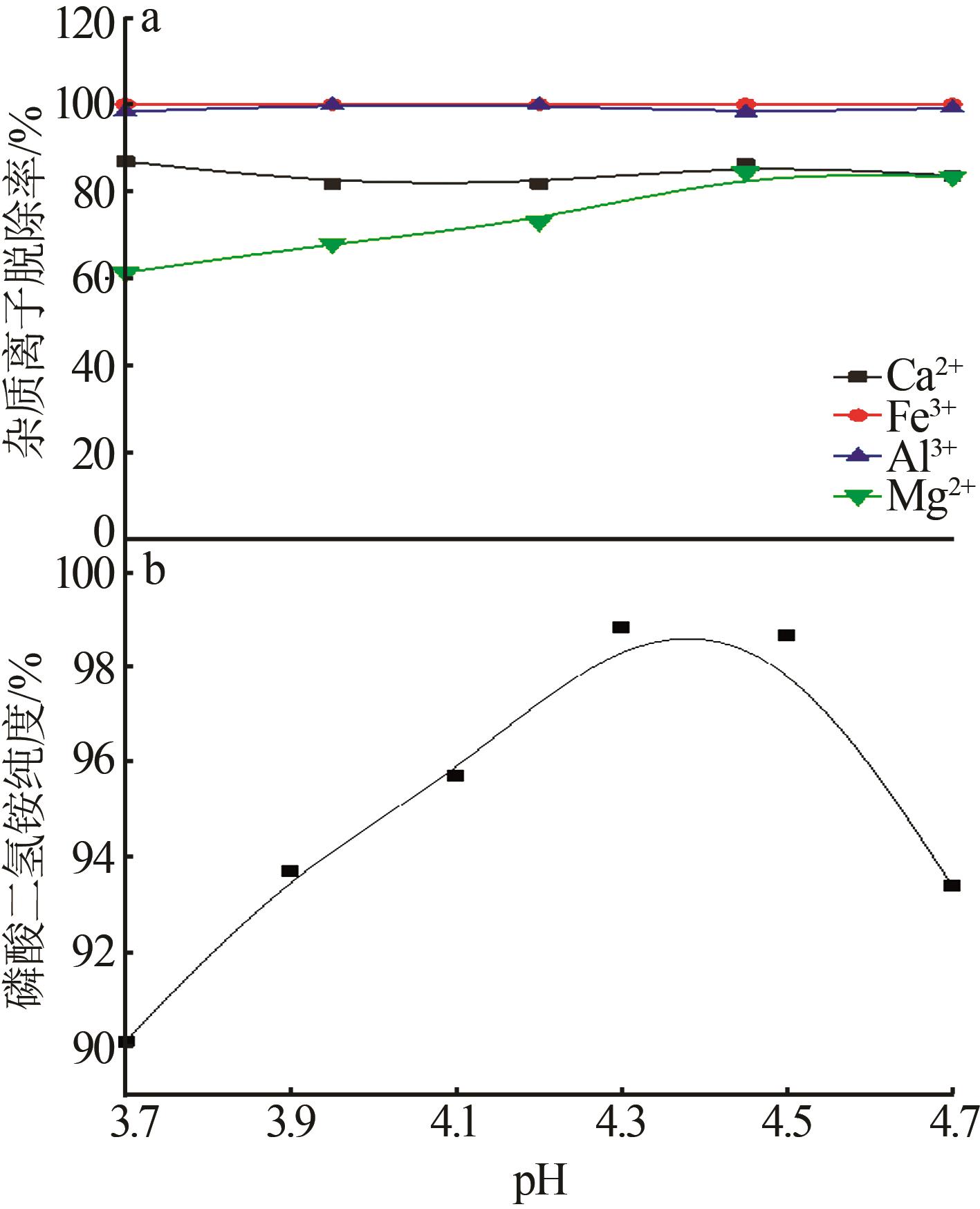
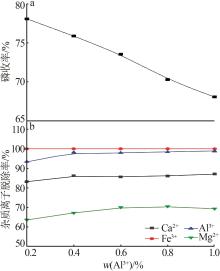
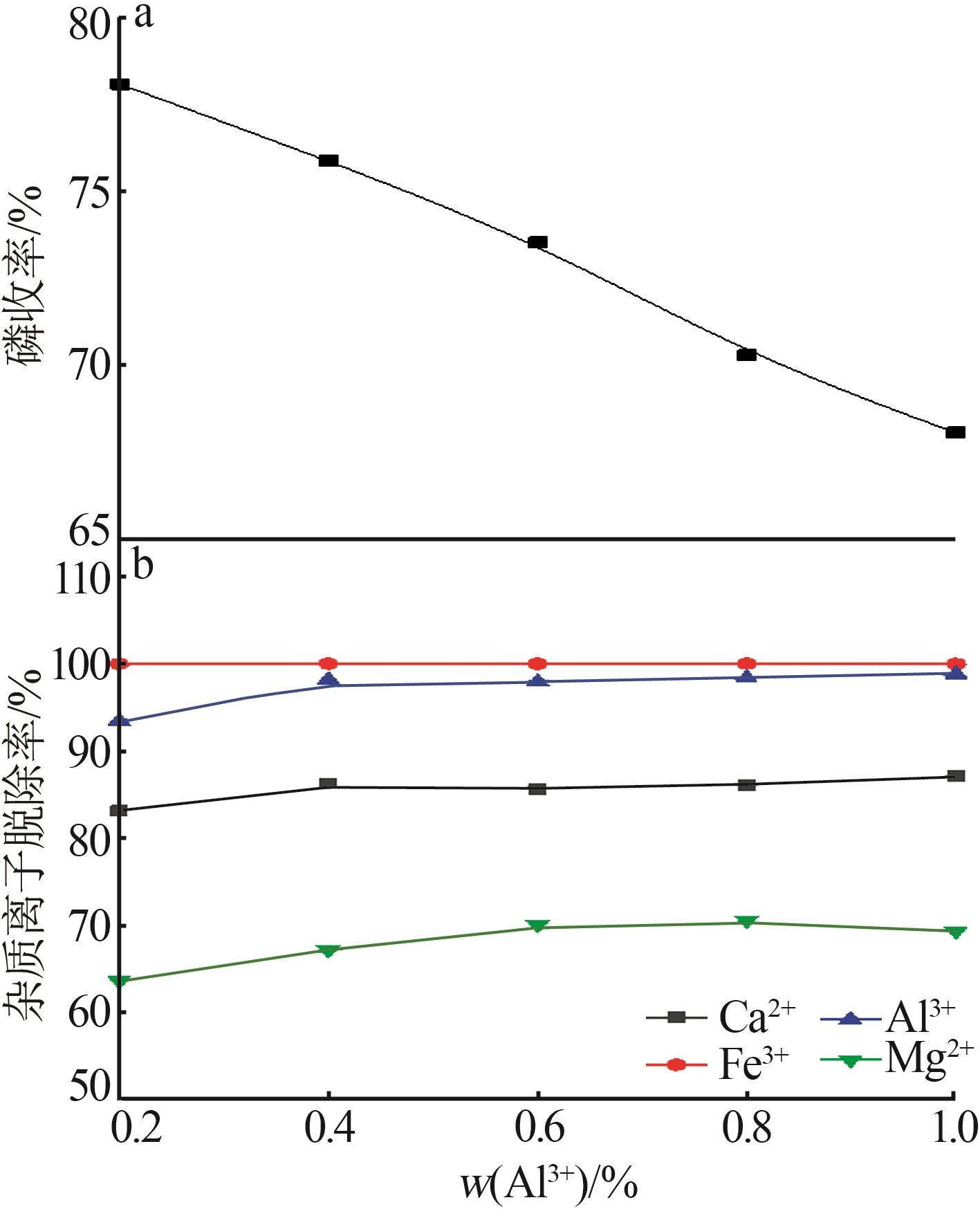
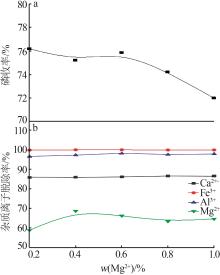
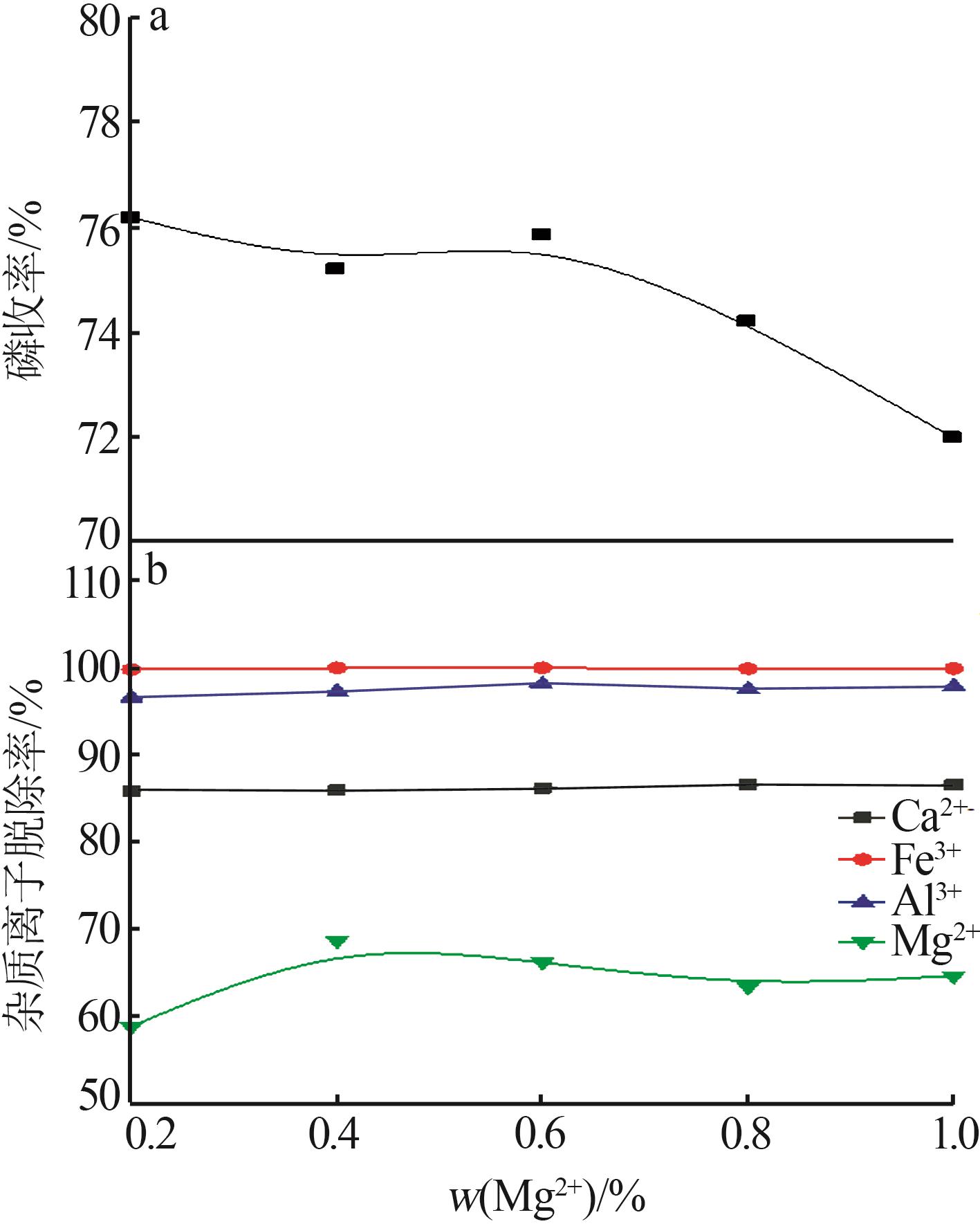
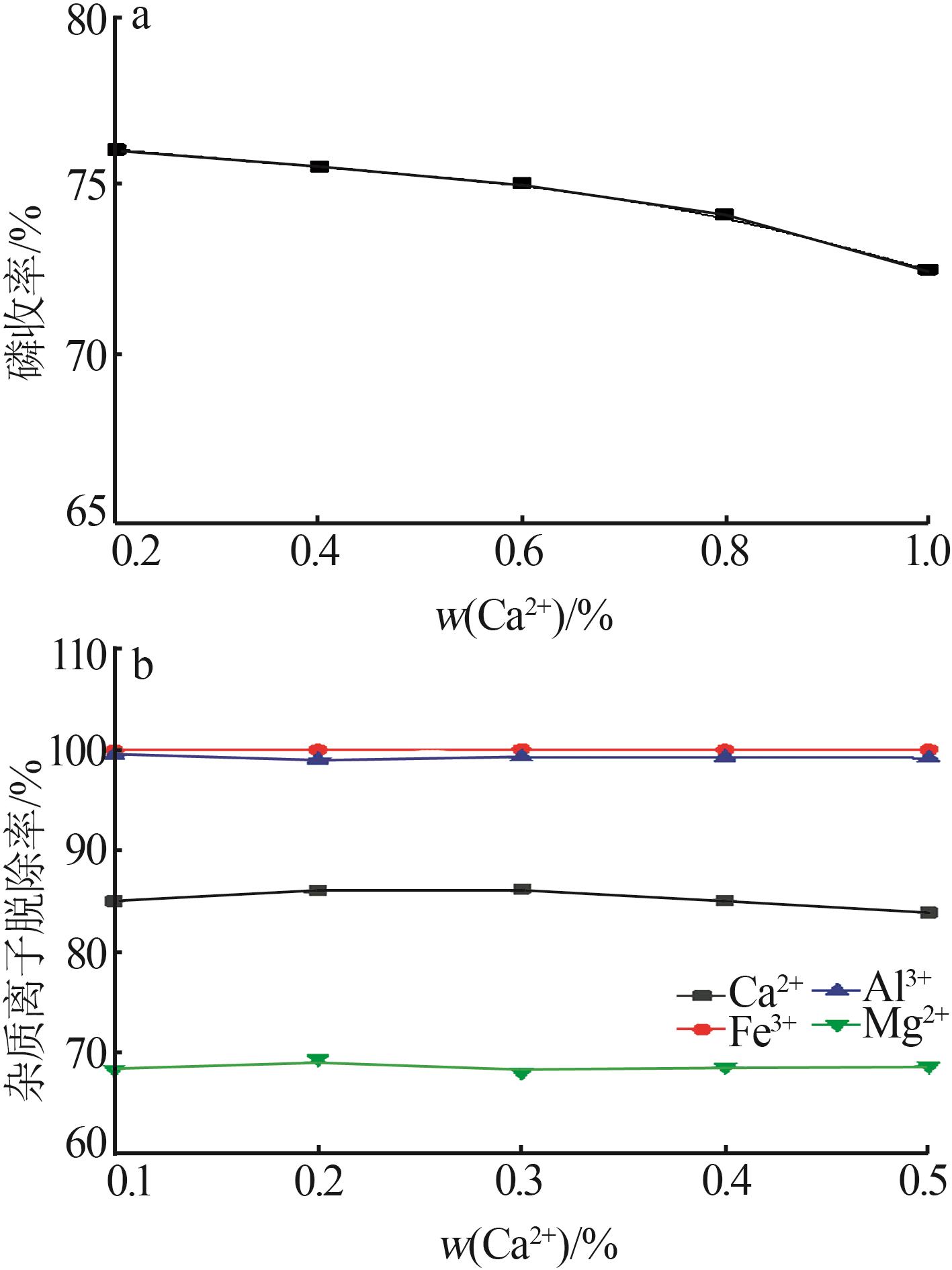
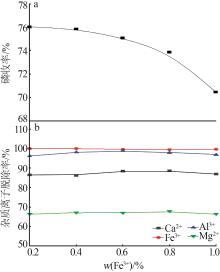
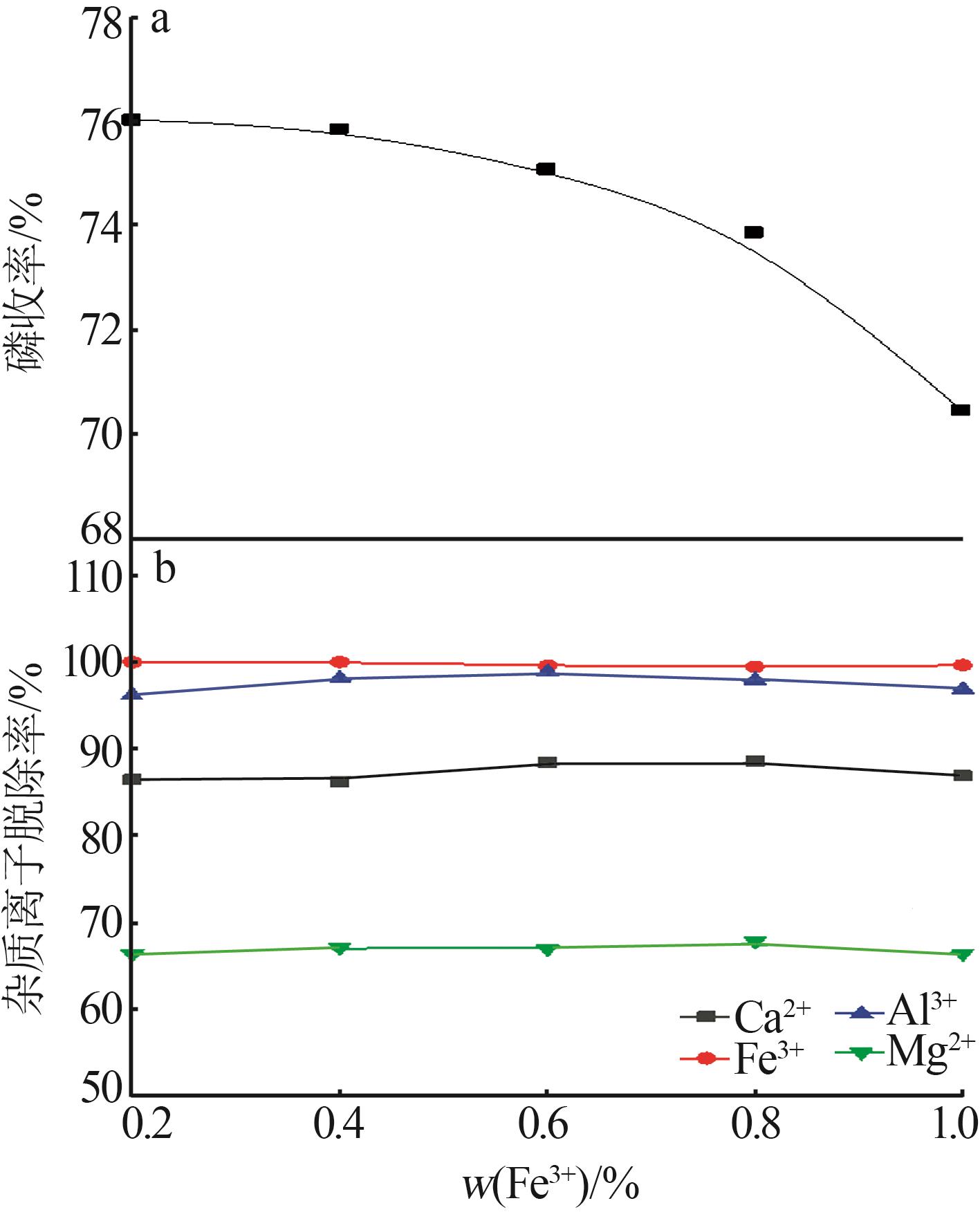
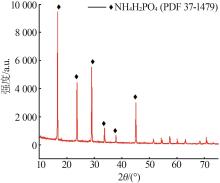
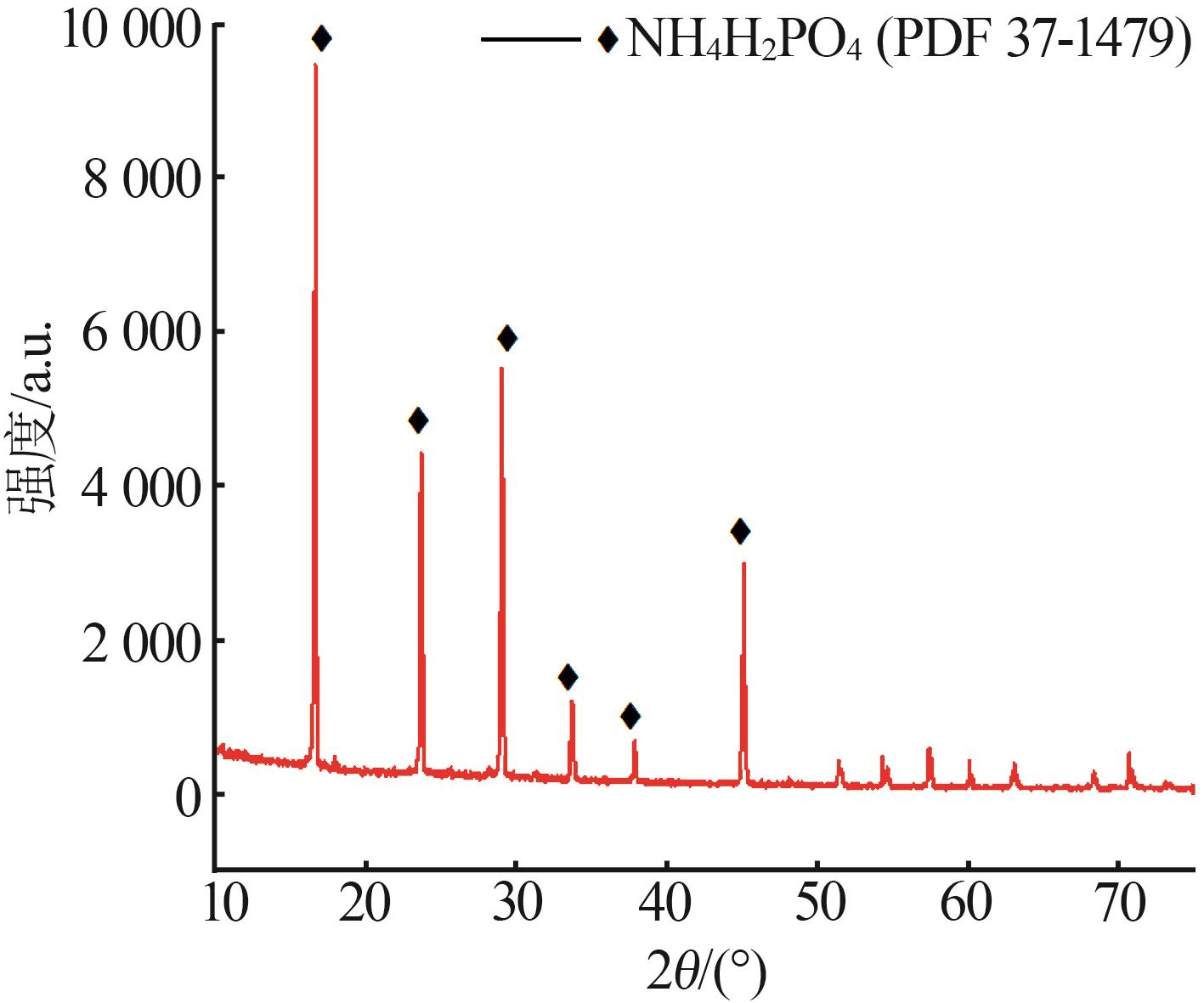
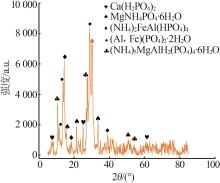
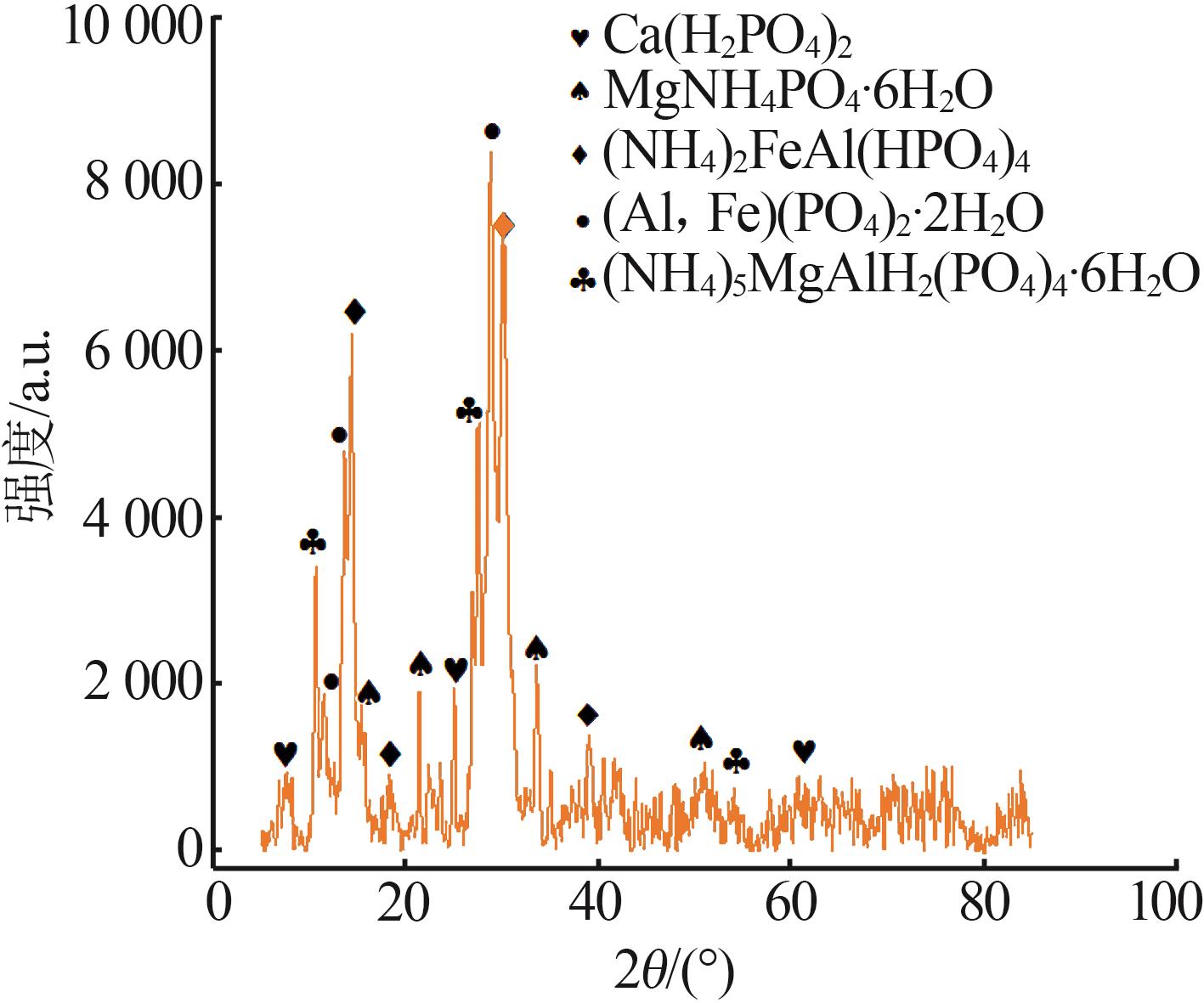
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (9): 50-56.doi: 10.19964/j.issn.1006-4990.2022-0686
• Research & Development • Previous Articles Next Articles
Research on process of production ammonium dihydrogen phosphate by ammonia purification of nitro-phosphoric acid
YUAN Jinghua( ), WU Junhu, YANG XiuShan(
), WU Junhu, YANG XiuShan( ), XU Dehua, ZHANG Zhiye
), XU Dehua, ZHANG Zhiye
- Ministry of Education Research Center for Comprehensive Utilization and Clean Processing Engineering of PhosphorusResources,College of Chemical Engineering,Sichuan University,Chengdu 610065,China
-
Received:2022-11-18Online:2023-09-10Published:2023-09-19
CLC Number:
Cite this article
YUAN Jinghua, WU Junhu, YANG XiuShan, XU Dehua, ZHANG Zhiye. Research on process of production ammonium dihydrogen phosphate by ammonia purification of nitro-phosphoric acid[J]. Inorganic Chemicals Industry, 2023, 55(9): 50-56.
share this article
| 1 | 周贵云,陈仕刚,蒲秋岑.湿法磷酸净化生产工业级磷酸一铵的技术现状及关键技术的工业应用[J].磷肥与复肥,2015,30(3):31-33. |
| ZHOU Guiyun, CHEN Shigang, PU Qiucen.Technical status of industrial grade monoammonium phosphate production by WPA purification method and industrial application of its key technology[J].Phosphate & Compound Fertilizer,2015,30(3):31-33. | |
| 2 | 崔飞,颜龙.磷-硼协效阻燃的云南松燃烧性能和热解动力学[J].中国安全科学学报,2018,28(7):38-44. |
| CUI Fei, YAN Long.Flame retardancy and pyrolysis kinetics of Pinus yunnanensis flame-retarded synergically by NH4H2PO4 and borax[J].China Safety Science Journal,2018,28(7):38-44. | |
| 3 | 王毕德,丁一刚,何俊,等.以三聚氰胺为缩合剂制备结晶Ⅱ型聚磷酸铵阻燃剂的工艺研究[J].应用化工,2017,46(10):1977-1980. |
| WANG Bide, DING Yigang, HE Jun,et al.Study on preparation of ammonium polyphosphate with crystalline form Ⅱ by melamine as condensing agent[J].Applied Chemical Industry,2017,46(10):1977-1980. | |
| 4 | 修学峰,白海丹.我国磷肥及大宗磷酸盐产品现状及发展浅析[J].磷肥与复肥,2014,29(3):1-6. |
| XIU Xuefeng, BAI Haidan.Status and development trend of phosphate fertilizer and bulk phosphate product in China[J].Phosphate & Compound Fertilizer,2014,29(3):1-6. | |
| 5 | 郑润,解田,刘飞,等.磷酸二氢铵应用研究进展[J].无机盐工业,2014,46(4):1-3. |
| ZHENG Run, XIE Tian, LIU Fei,et al.Research progress in application of ammonium dihydrogen phosphate[J].Inorganic Chemicals Industry,2014,46(4):1-3. | |
| 6 | 陈铭,娄伦武,卓知杰,等.湿法磷酸净化生产工业级磷酸一铵的工艺技术现状[J].化肥工业,2019,46(1):5-7,38. |
| CHEN Ming, LOU Lunwu, ZHUO Zhijie,et al.Current status of process technology for production of industrial grade monoammonium phosphate by wet phosphoric acid purification[J].Chemical Fertilizer Industry,2019,46(1):5-7,38. | |
| 7 | 王辛龙,许德华,钟艳君,等.中国磷化工行业60年发展历程及未来发展趋势[J].无机盐工业,2020,52(10):9-17. |
| WANG Xinlong, XU Dehua, ZHONG Yanjun,et al.Future trend and development course of phosphorus chemical industry for sixty years in China[J].Inorganic Chemicals Industry,2020,52(10):9-17. | |
| 8 | 王智娟,韦昌桃.工业级磷酸二氢铵生产工艺研究进展[J].无机盐工业,2021,53(6):118-122,198. |
| WANG Zhijuan, WEI Changtao.Research progress on production process of industrial grade ammonium dihydrogen phosphate[J].Inorganic Chemicals Industry,2021,53(6):118-122,198. | |
| 9 | 李新柱,郭宗端,贾亮,等.甲醇沉淀法净化湿法磷酸制备工业级磷酸一铵[J].无机盐工业,2016,48(2):40-41,67. |
| LI Xinzhu, GUO Zongduan, JIA Liang,et al.Preparation of industrial mono-ammonium phosphate from wet process phosphoric acid purified by methanol precipitation method[J].Inorganic Chemicals Industry,2016,48(2):40-41,67. | |
| 10 | 陈德清,王娜,张敏,等.湿法磷酸净化生产工业磷酸一铵研究[J].无机盐工业,2016,48(1):38-40. |
| CHEN Deqing, WANG Na, ZHANG Min,et al.Study on preparation of industrial grade MAP by WPA[J].Inorganic Chemicals Industry,2016,48(1):38-40. | |
| 11 | 李朝荣,苏殊,杨秀山,等.硝酸法湿法磷酸工艺的研究进展[C]//中国环境科学学会.2020年中国环境科学学会科学技术年会.南京:中国环境科学学会委员会: 557-561. |
| 12 | 吴子雄.用湿法磷酸生产高纯度磷酸一铵的工业装置[J].硫磷设计与粉体工程,2005(2):7-9,49. |
| WU Zixiong.Industrial plant for high purity monoammonium phosphate production with wet phosphoric acid[J].Sulphur Phosphorus & Bulk Materials Handling Related Engineering,2005(2):7-9,49. | |
| 13 | 韩磊.湿法磷酸氨化料浆渣制备磷酸铁[D].武汉:武汉工程大学,2018. |
| HAN Lei.Preparation of iron phosphate from ammoniated slurry residue of wet-process phosphoric acid[D].Wuhan:Wuhan Institute of Technology,2018. | |
| 14 | 王佩.湿法磷酸氨化料浆的絮凝沉降及其分离的研究[D].武汉:武汉工程大学,2017. |
| WANG Pei.Study on flocculation sedimentation and separation of ammoniated slurry of wet-process phosphoric acid[D].Wuhan:Wuhan Institute of Technology,2017. | |
| 15 | 荣圆.工业级磷酸一铵废渣的资源化研究[D].武汉:武汉工程大学,2018. |
| RONG Yuan.Study on resource utilization of waste residue in ammonium dihydrogenphosphate[D].Wuhan:Wuhan Institute of Technology,2018. | |
| 唐瑜钟,郑帅飞,张庆喜,等.铝及铝合金化学抛光磷酸废液制备磷酸二氢铵研究[J].无机盐工业,2021,53(5):88-92. | |
| TANG Yuzhong, ZHENG Shuaifei, ZHANG Qingxi,et al.Study on preparation of ammonium dihydrogen phosphate from phospho-ric acid waste solution of chemical polishing of aluminum and aluminum alloy[J].Inorganic Chemicals Industry,2021,53(5):88-92. | |
| 17 | 范益堃.萃余酸生产工业级磷酸一铵工艺优化及氨化数值模拟[D].武汉:武汉工程大学,2016. |
| FAN Yikun.Process optimization and ammoniation numerical simulation of ammonium dihydrogenphosphate production from raffinate acid.[D].Wuhan:Wuhan Institute of Technology,2016. |
| [1] | WANG Haosen, REN Bingchen, XU Dehua, YANG Xiushan, ZHANG Zhiye. Leaching process and kinetics of phosphorus-potassium ore decomposition by nitric acid [J]. Inorganic Chemicals Industry, 2023, 55(5): 45-51. |
| [2] | MA Dianpu, PU Youfu, LI Jun, CHEN Lishi, LIU Hengyu, QIN Deqing, FU Zewei, PENG Jubo. Preparation of high purity ultrafine tin dioxide particles by liquid oxidation-spray drying method [J]. Inorganic Chemicals Industry, 2023, 55(4): 54-59. |
| [3] | XIAO Yong,YANG Xiushan,XU Dehua,WANG Xinlong,ZHANG Zhiye. Study on treatment of medium and low grade high magnesium collophanite by nitric acid method [J]. Inorganic Chemicals Industry, 2022, 54(1): 71-76. |
| [4] | Su Shu,Xu Dehua,Li Chaorong,Yang Xiushan,Wang Xinlong,Zhang Zhiye. Study on defluorination of phosphoric acid by nitric acid process [J]. Inorganic Chemicals Industry, 2021, 53(9): 24-29. |
| [5] | Wang Zhijuan,Wei Changtao. Research progress on production process of industrial grade ammonium dihydrogen phosphate [J]. Inorganic Chemicals Industry, 2021, 53(6): 118-122. |
| [6] | Tang Yuzhong,Zheng Shuaifei,Zhang Qingxi,Xue Zhiqiang,Qin Jishan. Study on preparation of ammonium dihydrogen phosphate from phosphoric acid waste solution of chemical polishing of aluminum and aluminum alloy [J]. Inorganic Chemicals Industry, 2021, 53(5): 88-92. |
| [7] | Wang Zhijuan. Fabrication of industrial grade ammonium dihydrogen phosphate via selective removal of impurities from wet-process phosphoric acid [J]. Inorganic Chemicals Industry, 2021, 53(4): 48-51. |
| [8] | Yan Bo,Xie Tian,Zhu Huacheng,Yang Fengming,Wang Fengxia,Chen Yong. Study on process of synthesizing ammonium polyphosphate by microwave heating [J]. Inorganic Chemicals Industry, 2020, 52(7): 42-45. |
| [9] | Yang Ping,Yang Lin,Feng Yang,Lin Qian,Cao Jianxin. Migration and distribution of iodine in process of decomposing phosphate ore by nitric acid [J]. Inorganic Chemicals Industry, 2020, 52(4): 53-56. |
| [10] | WEN Yan-Bing, ZHANG Qin, GU Chun-Guang, LU Yu-Lian, ZHANG Lan-Xi, HAN Yu. Research progress on pretreatment technology of medium-and low-grade phosphate rock [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 7-. |
| [11] | XIONG Xing, ZHANG Bi-Jiang, XU De-Jun, ZHANG Zhi-Ye, RUAN Yong-Gang, YANG Lin, ZHOU Xue-Ke, WANG Xin-Long. Study on effects of cooling rate on ammonium dihydrogen phosphate′s metastable zone [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(6): 48-. |
| [12] | LUO Jin-Bo, WANG Hai-Feng, WANG Jia-Wei, ZHAO Ping-Yuan, ZHOU Ling-Ling. Experimental study on impurity removing from manganese anode slime by nitric acid pickling [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(4): 63-. |
| [13] | LIU Fei, JIE Tian, ZHENG Run, YANG Fan. Study on influencing factors of ammonium dihydrogen phosphate crystallization process [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(3): 46-. |
| [14] | XIAO Jing-Bo, HU Cai-Hua. Study on synthesis of boric acid and sodium nitrate by using borax mine in Tibet under assistance of nitric acid [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(5): 38-. |
| [15] | ZHENG Run, JIE Tian, LIU Fei, LI Tian-Xiang, ZHU Jing. Research progress in application of ammonium dihydrogen phosphate [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(4): 1-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||