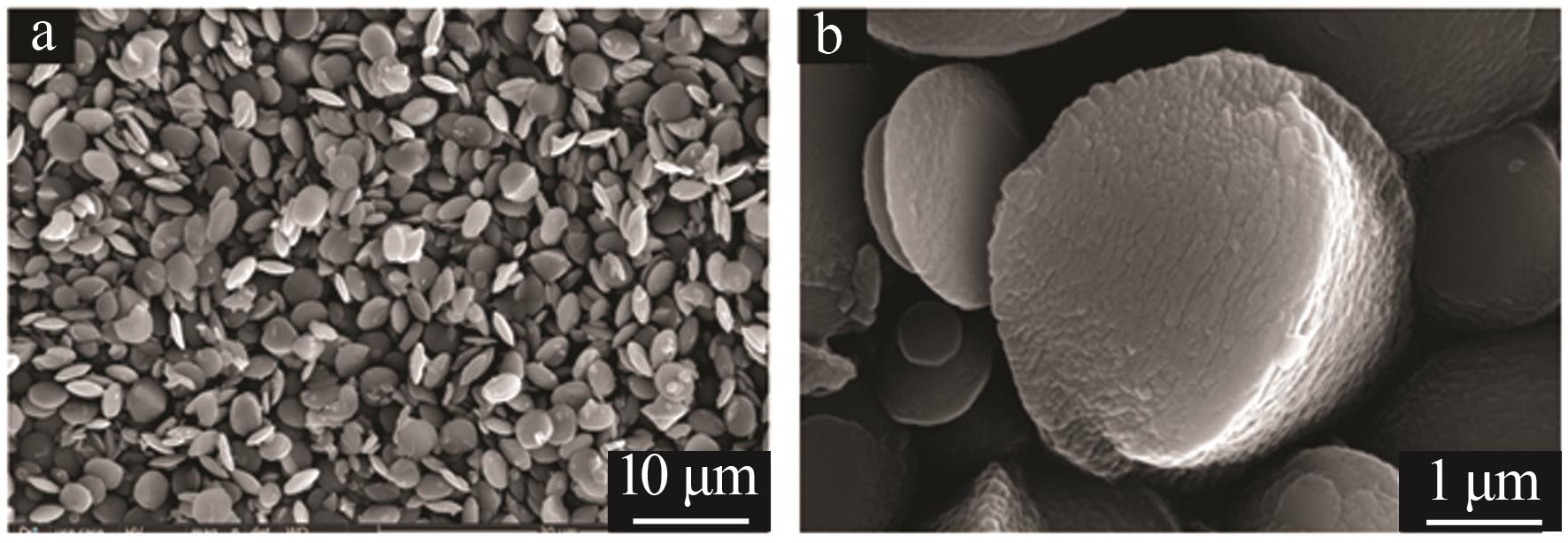
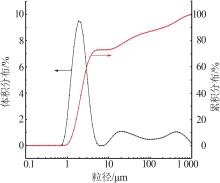
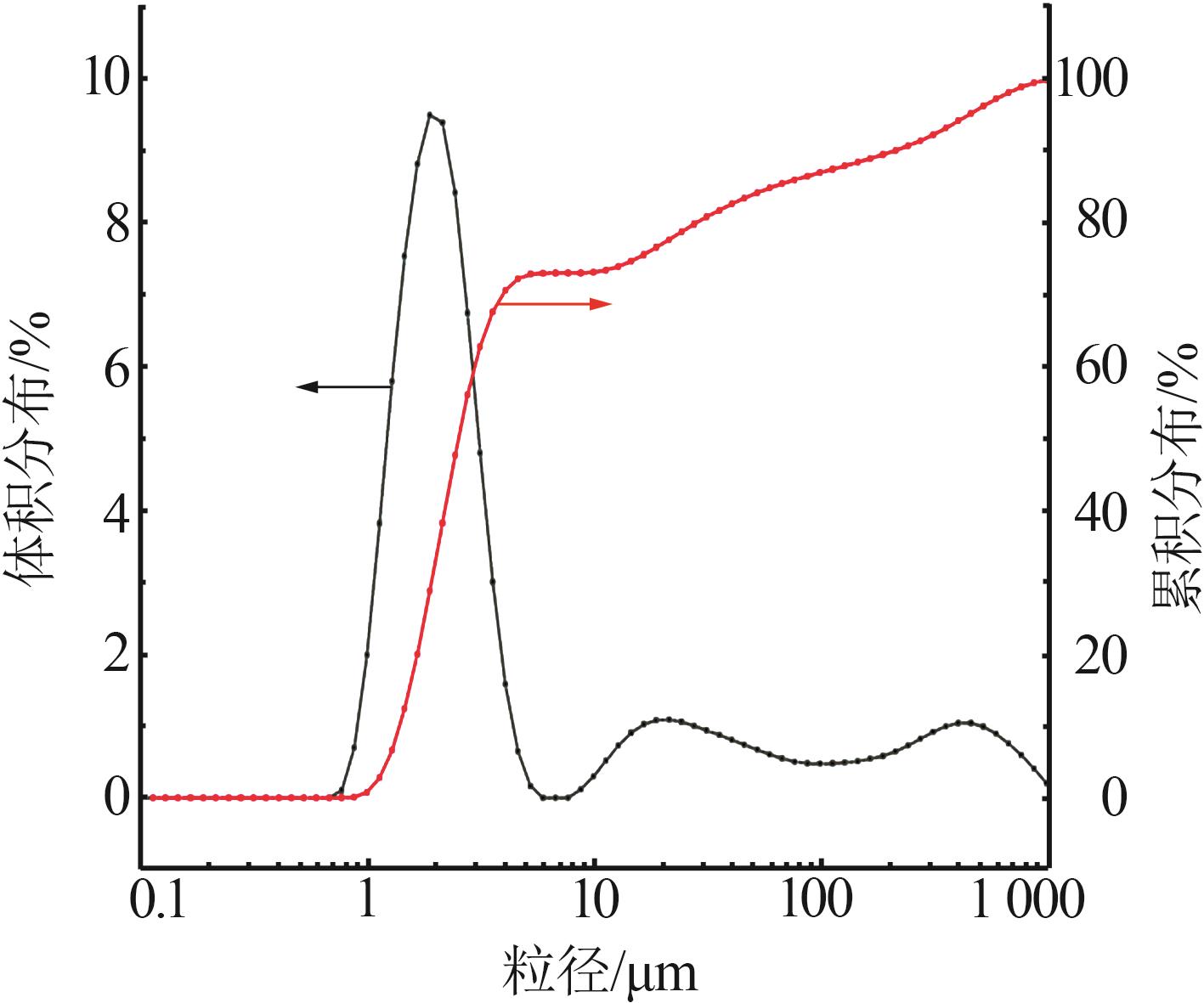
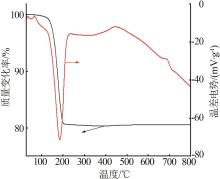
Inorganic Chemicals Industry ›› 2023, Vol. 55 ›› Issue (7): 51-57.doi: 10.19964/j.issn.1006-4990.2022-0548
• Research & Development • Previous Articles Next Articles
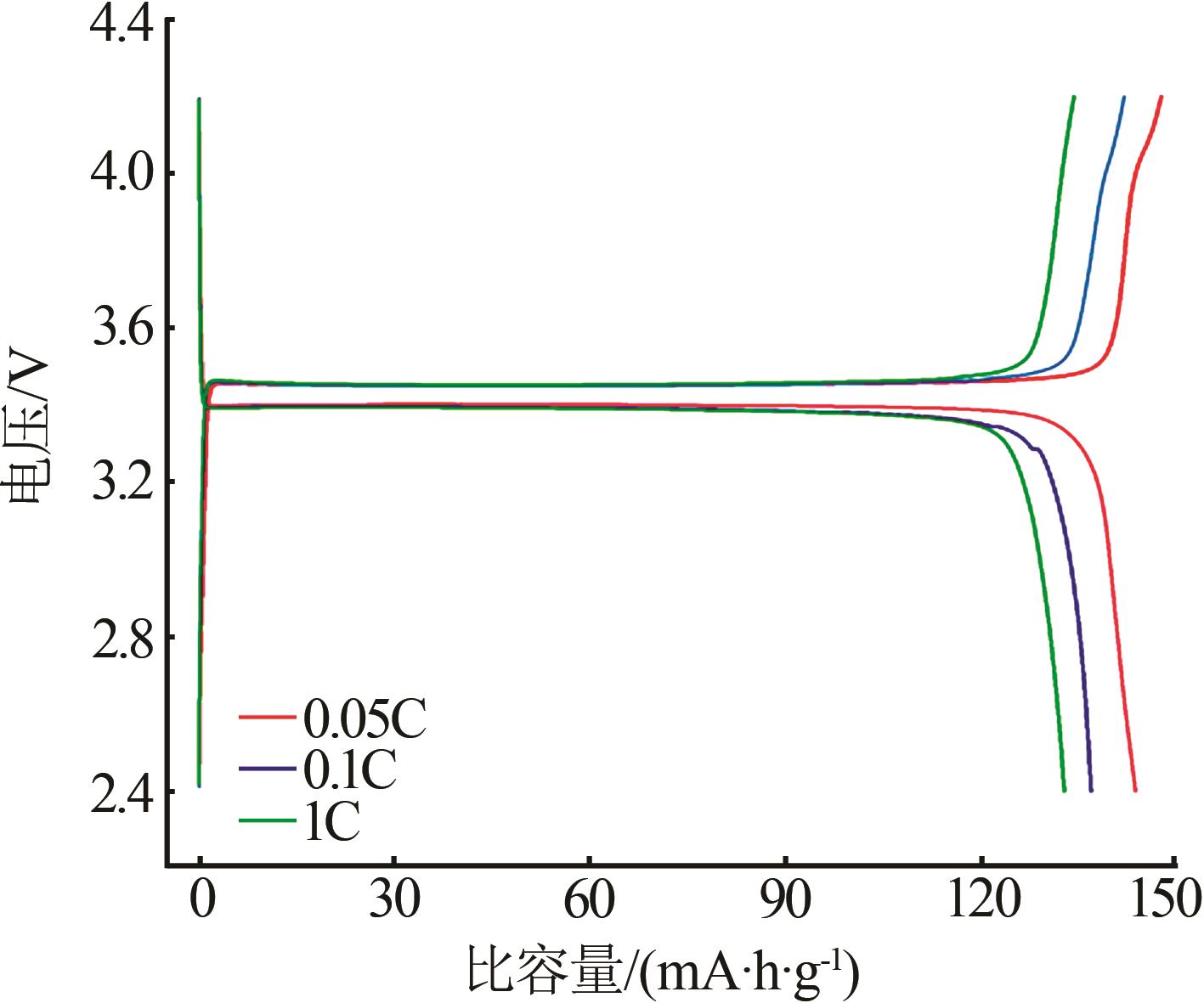
Study on preparation of battery grade ferric phosphate by co-precipitation of ferric nitrate and phosphoric acid
WANG Zihan( ), LI Jun, CHEN Ming, ZHOU Qingyu
), LI Jun, CHEN Ming, ZHOU Qingyu
- School of Chemical Engineering,Sichuan University,Chengdu 610065,China
-
Received:2022-09-11Online:2023-07-10Published:2023-07-13
CLC Number:
Cite this article
WANG Zihan, LI Jun, CHEN Ming, ZHOU Qingyu. Study on preparation of battery grade ferric phosphate by co-precipitation of ferric nitrate and phosphoric acid[J]. Inorganic Chemicals Industry, 2023, 55(7): 51-57.
share this article
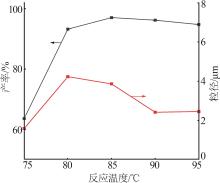
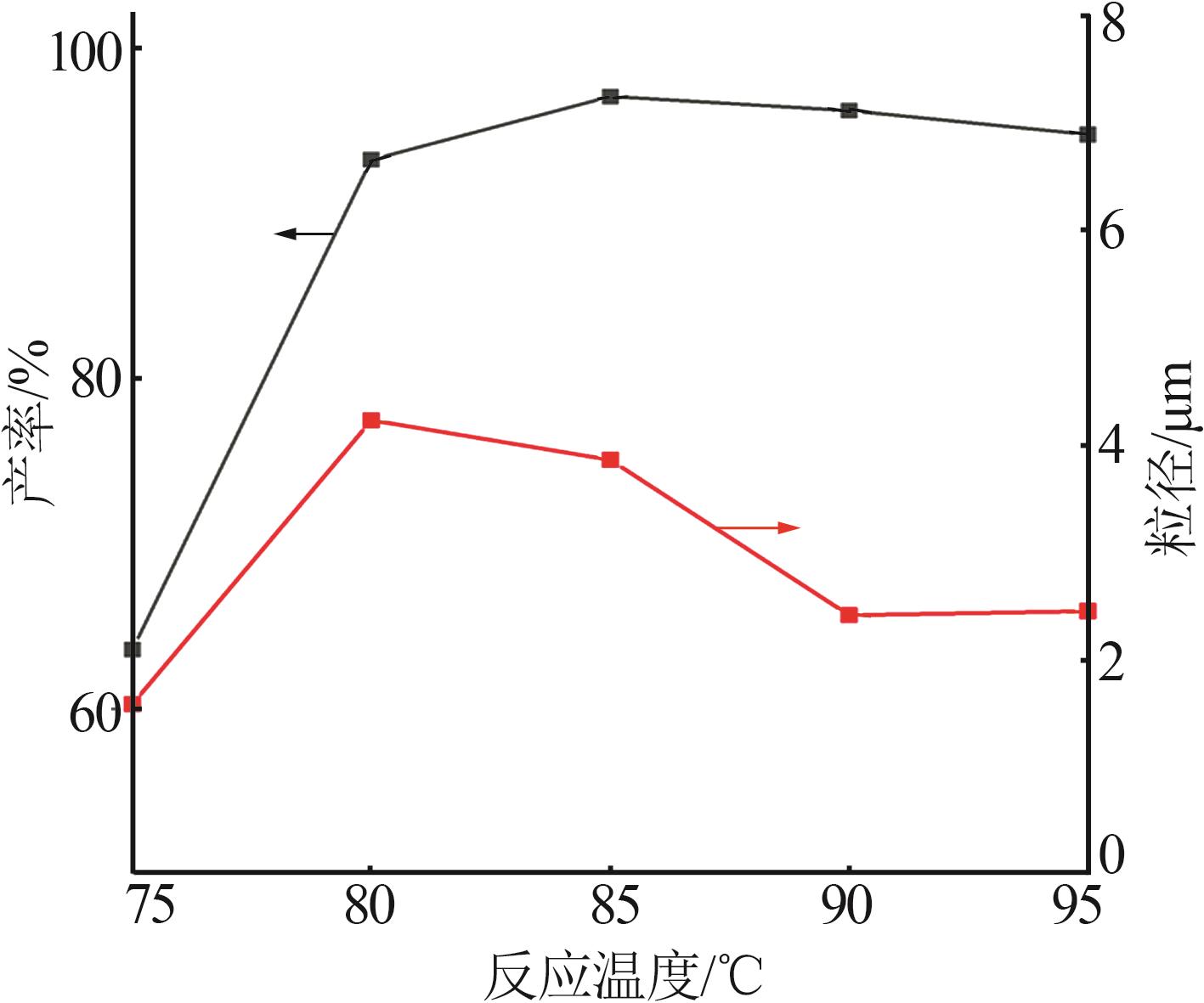
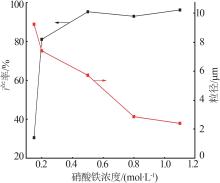
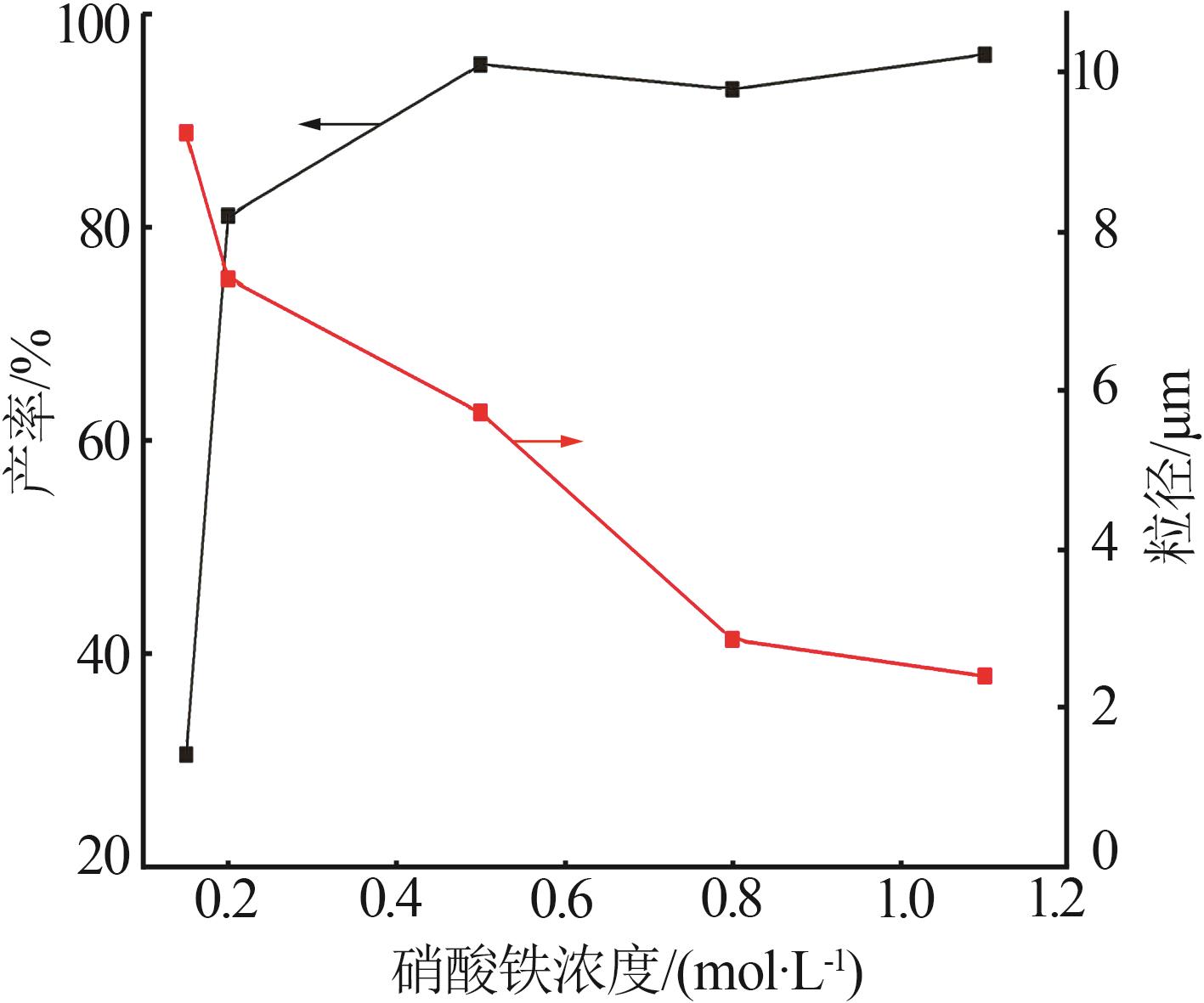
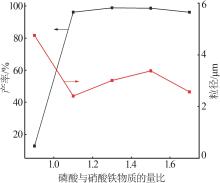
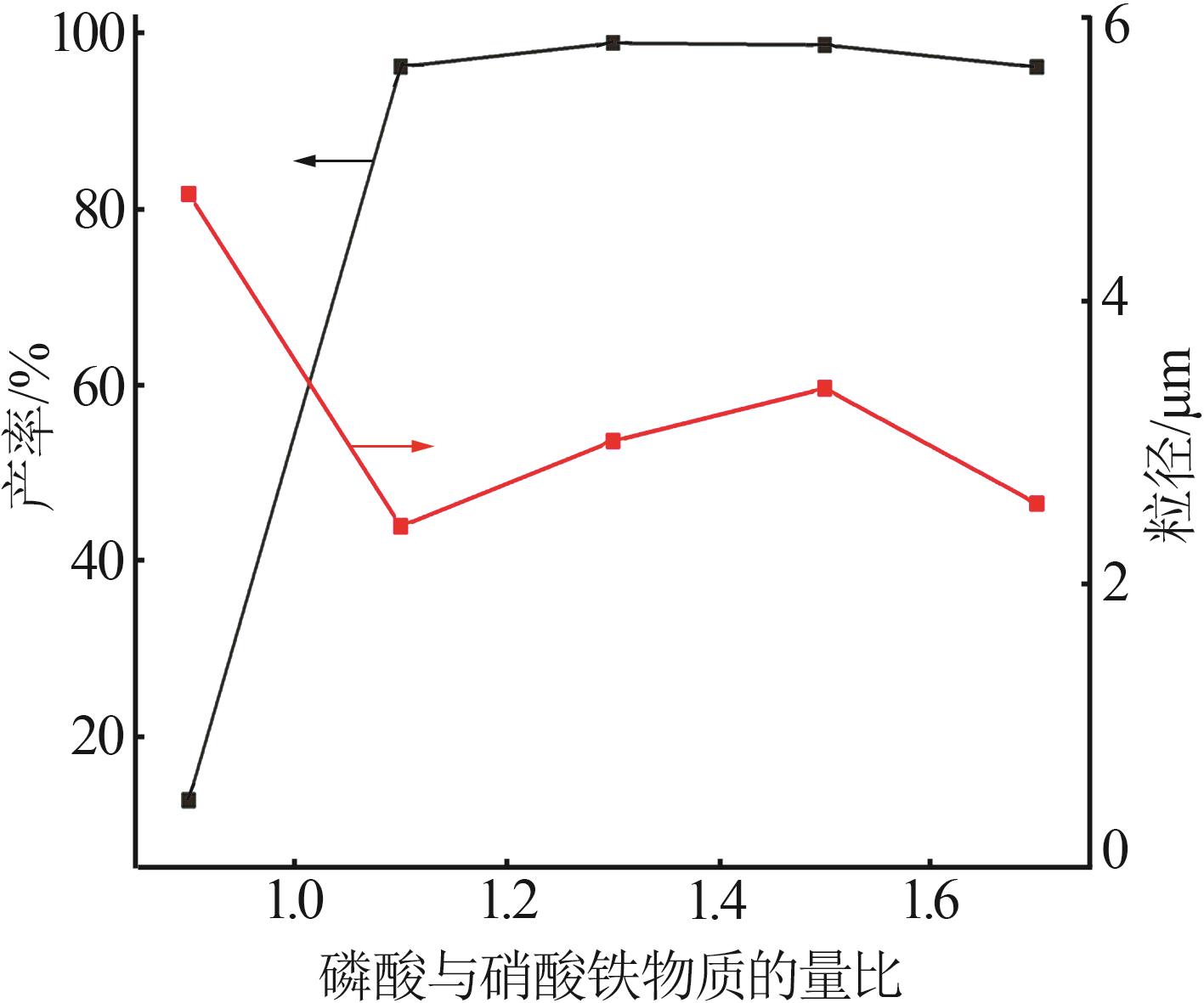
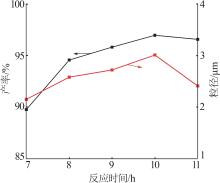
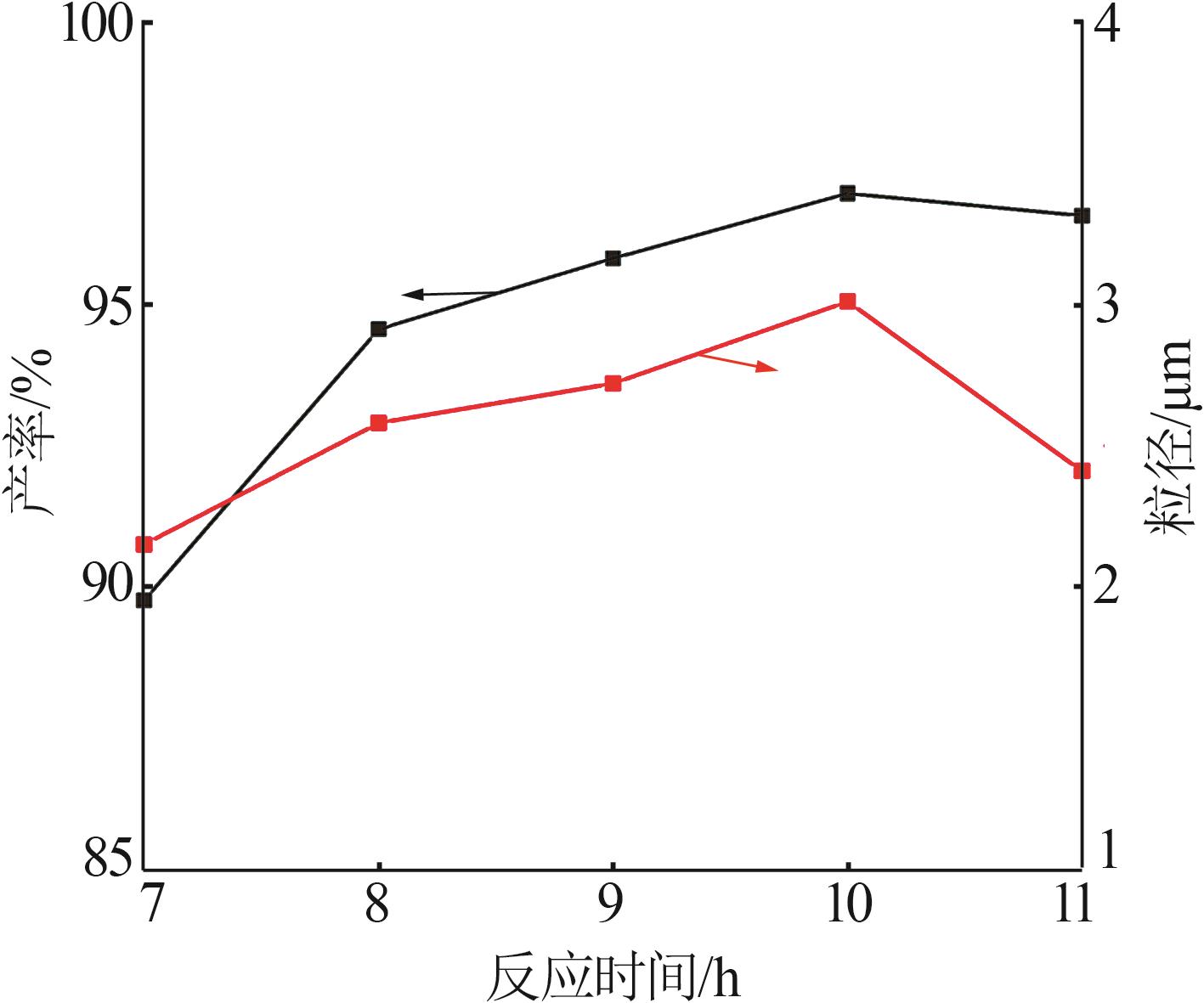
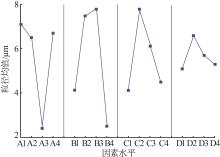
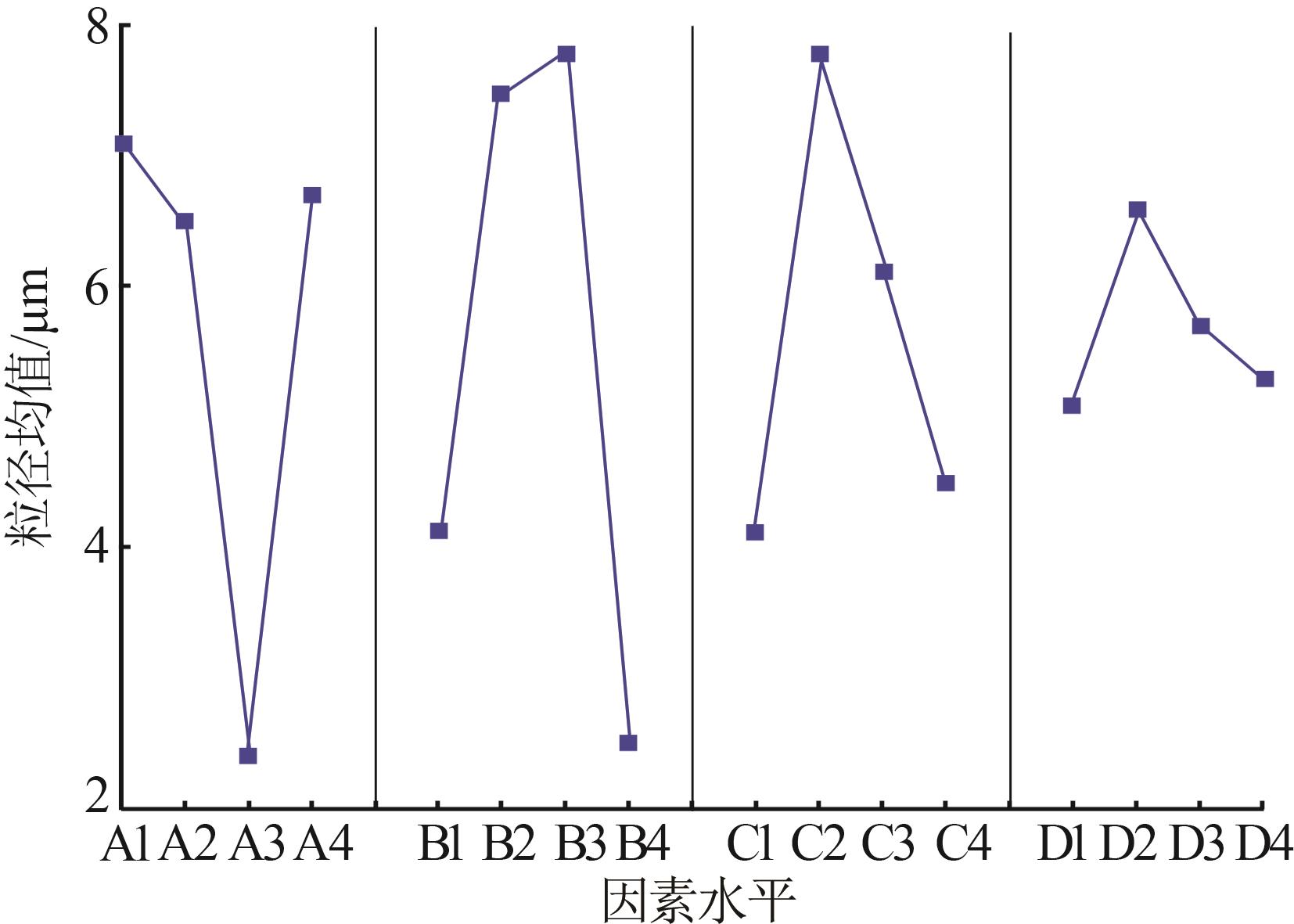
Table 2
Orthogonal experiment arrangement and results"
| 实验 | 实验因素 | 二次粒径/μm | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | 空白实验 | ||
| 1 | 1 | 1 | 1 | 1 | 1 | 4.23 |
| 2 | 1 | 2 | 2 | 2 | 2 | 12.2 |
| 3 | 1 | 3 | 3 | 3 | 3 | 9.12 |
| 4 | 1 | 4 | 4 | 4 | 4 | 2.52 |
| 5 | 2 | 1 | 2 | 4 | 3 | 5.95 |
| 6 | 2 | 2 | 1 | 3 | 4 | 6.98 |
| 7 | 2 | 3 | 4 | 2 | 1 | 9.23 |
| 8 | 2 | 4 | 3 | 1 | 2 | 3.86 |
| 9 | 3 | 1 | 3 | 2 | 4 | 2.88 |
| 10 | 3 | 2 | 4 | 1 | 3 | 2.58 |
| 11 | 3 | 3 | 1 | 4 | 2 | 2.87 |
| 12 | 3 | 4 | 2 | 3 | 1 | 3.01 |
| 13 | 4 | 1 | 4 | 3 | 2 | 3.83 |
| 14 | 4 | 2 | 3 | 4 | 1 | 9.06 |
| 15 | 4 | 3 | 2 | 1 | 4 | 10.7 |
| 16 | 4 | 4 | 1 | 2 | 3 | 2.56 |
| K1 | 28.07 | 16.89 | 16.64 | 21.37 | 25.53 | |
| K2 | 26.02 | 30.82 | 31.86 | 26.87 | 22.76 | |
| K3 | 11.34 | 31.92 | 24.92 | 22.94 | 20.21 | |
| K4 | 26.15 | 11.95 | 18.16 | 20.40 | 23.08 | |
| k1 | 7.02 | 4.22 | 4.16 | 5.34 | 6.38 | |
| k2 | 6.51 | 7.71 | 7.97 | 6.72 | 5.69 | |
| k3 | 2.84 | 7.98 | 6.23 | 5.74 | 5.05 | |
| k4 | 6.54 | 2.99 | 4.54 | 5.10 | 5.77 | |
| R | 4.18 | 4.99 | 3.81 | 1.62 | 1.33 | |
| 1 | 张克杰,陈鹏,谢旺旺,等.磷酸铁锂优缺点及改性研究进展[J].无机盐工业,2018,50(6):13-17. |
| ZHANG Kejie, CHEN Peng, XIE Wangwang,et al.Progress in modification of lithium iron phosphate and its advantages and disadvantages[J].Inorganic Chemicals Industry,2018,50(6):13-17. | |
| 2 | 梁广川.锂离子电池用磷酸铁锂正极材料[M].北京:科学出版社,2013:288-292. |
| 3 | 张婷,林森,于建国.磷酸铁锂正极材料的制备及性能强化研究进展[J].无机盐工业,2021,53(6):31-40. |
| ZHANG Ting, LIN Sen, YU Jianguo.Research progress in synthesis and performance enhancement of LiFePO4 cathode materials[J].Inorganic Chemicals Industry,2021,53(6):31-40. | |
| 4 | 刘贡钢,叶红齐,刘辉,等.前驱体磷酸铁的制备及其对磷酸 |
| 铁锂电化学性能的影响[J].应用化工,2013,42(2):225- 228. | |
| LIU Gonggang, YE Hongqi, LIU Hui,et al.Preparation of precursor FePO4 and its effects on electrochemical properties of LiFePO4 [J].Applied Chemical Industry,2013,42(2):225-228. | |
| 5 | 袁静楠.不同晶型磷酸铁的制备及与磷酸亚铁锂间的相互转换关系[D].郑州:郑州大学,2012. |
| YUAN Jingnan.Preparation of different crystal forms of iron phosphate and their conversion relationship with lithium ferrous phosphate[D].Zhengzhou:Zhengzhou University,2012. | |
| 6 | 姚停,徐婷,宋磊,等.磷酸铁形貌对磷酸铁锂倍率性能的影响[J].电池,2020,50(1):54-57. |
| YAO Ting, XU Ting, SONG Lei,et al.Effects of morphology of ferric phosphate on rate capability of lithium iron phosphate[J].Battery Bimonthly,2020,50(1):54-57. | |
| 7 | 吴康,李军,陈明.乙醇-水体系3D纳/微米球形磷酸铁的制备与表征[J].无机盐工业,2020,52(6):41-45. |
| WU Kang, LI Jun, CHEN Ming.Synthesis and characterization of 3D nano/micro spherical iron phosphate in ethanol-water system[J].Inorganic Chemicals Industry,2020,52(6):41-45. | |
| 8 | 马志鸣,肖仁贵,廖霞,等.片层纳米结构磷酸铁制备及对磷酸铁锂电性能的影响[J].材料导报,2018,32(19):3325-3331. |
| MA Zhiming, XIAO Rengui, LIAO Xia,et al.Preparation of the lameller nanostructure iron phosphate and its effect on the electrochemical performance of lithium iron phosphate[J].Materials Review,2018,32(19):3325-3331. | |
| 9 | 赵曼.水热法以磷铁制备电池级磷酸铁及改性研究[D].贵阳:贵州大学,2017. |
| ZHAO Man.Preparation of battery-grade iron phosphate from ferrophosphorus by hydrothermal method and its modification[D].Guiyang:Guizhou University,2017. | |
| 10 | 严鹏,谢刚,张春明,等.非晶态磷酸铁的沉淀反应机理及性能[J].电池,2015,45(5):241-243. |
| YAN Peng, XIE Gang, ZHANG Chunming,et al.Precipitation reaction mechanism and performance of amorphous ferric phosphate[J].Battery Bimonthly,2015,45(5):241-243. | |
| 11 | 张震,蒲薇华,任建国,等.控制结晶法制备球形磷酸铁的团聚尺寸模型[J].化学工程,2011,39(8):20-24. |
| ZHANG Zhen, PU Weihua, REN Jianguo,et al.Agglomeration-size model of ferric phosphate spherical particle prepared by controlled crystallization[J].Chemical Engineering(China),2011,39(8):20-24. | |
| 12 | 李永佳,魏润宏,鲁劲华,等.电池级磷酸铁的制备及性能[J].化工进展,2021,40(4):2227-2233. |
| LI Yongjia, WEI Runhong, LU Jinhua,et al.Preparation and performance of FePO4 precursor for LiFePO4 [J].Chemical Industry and Engineering Progress,2021,40(4):2227-2233. | |
| 13 | 郑典模,潘鹤政,伍丽萍,等.超细磷酸铁的制备及其电化学性能研究[J].电源技术,2015,39(1):58-61. |
| ZHENG Dianmo, PAN Hezheng, WU Liping,et al.Study on preparation and electrochemical of ultrafine FePO4 [J].Chinese Journal of Power Sources,2015,39(1):58-61. | |
| 14 | 李文升,樊勇利,童书辉,等.高振密球形FePO4·xH2O的合成研究[J].电源技术,2013,37(6):950-952. |
| LI Wensheng, FAN Yongli, TONG Shuhui,et al.Synthesis research on iron phosphate with spherical structure and high tap density[J].Chinese Journal of Power Sources,2013,37(6):950-952. | |
| 15 | ZHANG Tongbao, XIN Dawei, LU Yangcheng,et al.Direct precipitation for a continuous synthesis of nanoiron phosphate with high purity[J].Industrial & Engineering Chemistry Research,2014,53(16):6723-6729. |
| [1] | PAN Xiaoxiao, ZHUANG Shuxin, SUN Yuqing, SUN Gaoxing, REN Yan, JIANG Shengyu. Research progress of modified-LiFePO4 as cathode materials for lithium ion batteries [J]. Inorganic Chemicals Industry, 2023, 55(6): 18-26. |
| [2] | CHEN Yujue, ZHANG Liangjun, KUANG Huan, JIANG Manwen, XIAO Li. Study on roasting and recovery process of waste lithium iron phosphate powder with sodium bisulfate [J]. Inorganic Chemicals Industry, 2023, 55(3): 113-117. |
| [3] | CHEN Zhangxu, FU Minglian, ZHU Danchen, ZHENG Bingyun. Preparation of carbon/graphite carbon nitride composites and their methylene blue removal performance [J]. Inorganic Chemicals Industry, 2023, 55(3): 134-139. |
| [4] | GONG Jiazhu,ZHOU Guimin,WU Ninglan,WANG Weilin,QIAO Guangqin. Challenges and innovative development opportunities of carbon peak and carbon neutralization faced by inorganic salt industry [J]. Inorganic Chemicals Industry, 2022, 54(4): 46-54. |
| [5] | YANG Wenyu,LIN Zhiya,FU Hong,YAN Wenyue,LIN Jianping,GUAN Guiqing. Study on surface potential of lithium iron phosphate based on kelvin probe technology [J]. Inorganic Chemicals Industry, 2022, 54(11): 65-70. |
| [6] | Zhang Ting,Lin Sen,Yu Jianguo. Research progress in synthesis and performance enhancement of LiFePO4 cathode materials [J]. Inorganic Chemicals Industry, 2021, 53(6): 31-40. |
| [7] | HE Yanjun,ZHENG Hanxiao,LÜ Li,TANG Shengwei. Carbothermal reduction process of low-grade mixed phosphate ore [J]. Inorganic Chemicals Industry, 2021, 53(11): 95-99. |
| [8] | Liu Peiwen,Dong Peng,Meng Qi,Yang Xuan,Zhou Siyuan. Research development of solid phase regeneration of cathode material of spent lithium iron phosphate batteries [J]. Inorganic Chemicals Industry, 2020, 52(9): 6-8. |
| [9] | Guo Ju,Jia Shuangzhu. Study on the one-step hydrothermal synthesis of LiFePO4 and its properties [J]. Inorganic Chemicals Industry, 2020, 52(6): 36-40. |
| [10] | Wang Jiatai,Zhao Duan,Ma Lianhua,Zhang Caihong. Research progress of LiFePO4 cathode materials for Li-ion battery [J]. Inorganic Chemicals Industry, 2020, 52(4): 18-22. |
| [11] | Li Jieen,He Binbin,Yang Xiushan,Xu Dehua,Zhang Zhiye. Study on phosphorus extraction and impurity reduction technology for pretreatment of middle-low grade phosphate ore [J]. Inorganic Chemicals Industry, 2020, 52(4): 33-36. |
| [12] | Su Wei,Zhang Linfeng,Lü Qingyang,Xia Yuyan,Yuan Hua. Synthesis of diphenyl carbonate by oxidative carbonylation over palladium loaded MnOx-based bimetallic oxide catalysis [J]. Inorganic Chemicals Industry, 2019, 51(9): 91-96. |
| [13] | Chen Lei,Zhao Longtao,Chen Zhenyu,Li Guang. Preparation of spherical lithium iron phosphate material and its 18650 battery test [J]. Inorganic Chemicals Industry, 2019, 51(8): 25-28. |
| [14] | BIAN Du-Cheng, LIU Shu-Lin, TIAN Yuan. Reusing of spent LiFePO4 cathode materials by solid phase lithium refilling method and electrochemical performance there of [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 71-. |
| [15] | MAO Jia-Yu, XIAO Yang. Research progress in doping modification of LiFePO4/C cathode materials [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(1): 13-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||