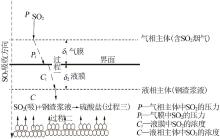
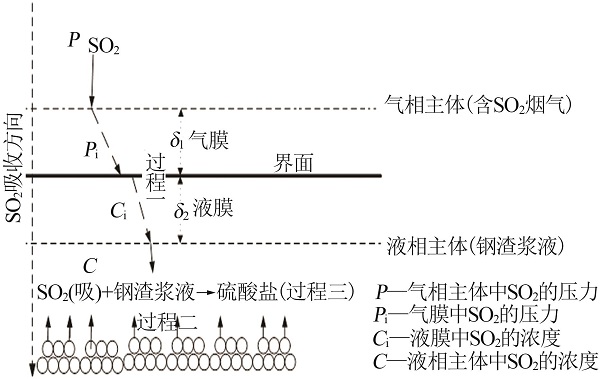
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (11): 39-44.doi: 10.19964/j.issn.1006-4990.2021-0778
• Reviews and Special Topics • Previous Articles Next Articles
Research progress on flue gas desulfurization by modification of steel slag
DU Xiaoyan1,2( ),LONG Hongming2,LIU Xiuyu1,ZHU Qingming1,HAN Weisheng1
),LONG Hongming2,LIU Xiuyu1,ZHU Qingming1,HAN Weisheng1
- 1. School of Civil Engineering and Architecture,Anhui University of Technology,Ma'anshan 243032,China
2. State Key Laboratory of Metallurgical Emission Reduction & Resources Recycling(Anhui University of Technology)
-
Received:2021-12-27Online:2022-11-10Published:2022-11-23
CLC Number:
Cite this article
DU Xiaoyan,LONG Hongming,LIU Xiuyu,ZHU Qingming,HAN Weisheng. Research progress on flue gas desulfurization by modification of steel slag[J]. Inorganic Chemicals Industry, 2022, 54(11): 39-44.
share this article
Table 1
Research on desulfurization of modified steel slag"
| 改性方式 | 改性途径 | 组分,质量分数/% | 反应器 | 反应条件 | 脱硫率/% |
|---|---|---|---|---|---|
| 物理改性 | 粉磨至75 μm | CaO:45~50;MgO:4~5;Fe2O3:7~10;FeO:10~18;SiO2:10~11;MnO:0.5~2.5;Al2O3: 1~4;P2O5:3~5;f-CaO:5~11 | 填料塔 | pH=8~11;液气比为10~24 L/m3;浆液质量分数为4% | 60[ |
| 粒度<45 μm | CaO:38.02;MgO:7.62;FeO:9.3;SiO2:12.47;Fe:15.92;MnO:2.63;Al2O3:6.99 | 吸收塔 | 烟气流量为34.84万m3/h;入口SO2质量浓度 为7 089.85 mg/m3 | 98.16[ | |
| 粒度<75 μm | CaO:59.16;MgO:3.22;Fe2O3:10.75;SiO2:12.79;SO3:0.77;MnO:3.22;Al2O3:5.89;其 他:6.85 | 鼓泡 反应器 | 反应温度为30 ℃、浆液质量分数为8%;烟气 流量为400 mL/min | 95[ | |
| 比表面积=400 m2/kg | CaO:52.35;MgO:10.85;Fe:4.91;S:0.294 | 循环 硫化床 | 入口SO2质量浓度为900 mg/m3;钢渣加入量 为1 300 kg/h;喷水流量为360 L/min | 72[ | |
| 粉磨至75 μm | CaO:>45 | 再生池 | 钢渣加入量为40 g;入口SO2质量浓度为 2 400 mg/m3;烟气流量为0.05 m3/h;pH=7~8 | 80[ | |
| 化学改性 | 碱式碳酸镁与钢渣按10∶1质量比混合 | CaO:46.83;MgO:7.61;Fe2O3:1.24;SiO2: 32.28;MnO:1.00;Al2O3:8.73 | 鼓泡反 应装置 | 反应温度为100 ℃;搅拌速率为300 r/min;质量 比为10∶1;粒径≥0.074 mm;反应时间<150 min | 80[ |
粒径为75 μm钢渣与2 mmol/L 柠檬酸反应2 h | CaO:43.25;Fe2O3:14.99;SiO2:22.85;Al2O3:6.32;MnO:2.87; MgO:4.26;P2O5:1.9 | 鼓泡 反应器 | 钢渣浆液质量分数为4%;入口SO2质量浓度为1 500 mg/m3 | 90[ | |
| 粒径为75 μm钢渣与质量分数为27%H2SO4混合,调节至浆液pH为5.5~6.0 | CaO:41.38;MgO:6.5;Fe2O3:17.67;SiO2: 17.62;SO3:1.17;MnO:1.13;Al2O3:8.16; MnO:1.13 | 鼓泡搅 拌装置 | 反应温度为40 ℃;浆液pH=5.5;氧化度OR=0.8 | 100[ | |
| 10 μm钢渣与活性炭按质量比1∶2混合,以400 r/min 速率在行星式球磨机中机械搅拌1 h | SiO2:44.90;CaO:21.16;Al2O3:15.76; Fe2O3:11.10;MgO:2.41 | 固定床 反应器 | 入口SO2体积分数为0.06%;气体流量为 600 mL/min;吸附剂质量为6 g;体积空速为 3 600 h-1;反应温度为120 ℃ | 79[ | |
| 75 μm钢渣与水菱镁按质量比 10∶1混合,在300 r/min速率下搅拌一定时间 | — | 鼓泡器 | 入口SO2质量浓度为2 000 mg/m3;浆液pH= 5;浆液浓度为0.5 mol/L;烟气流量为 50 mL/min;反应温度为60 ℃;搅拌速率为 100 r/min;鼓泡深度为2.5 cm | 98.55[ |
| 1 | 杨丽韫,陈军,袁鹏,等.钢渣去除废水中重金属离子的研究综述[J].钢铁,2017,52(8):1-9. |
| YANG Liyun, CHEN Jun, YUAN Peng, et al.Research review of heavy metal ions removal from waste water by steelmaking slag[J].Iron & Steel,2017,52(8):1-9. | |
| 2 | 张顺雨.钢渣成分在烟气脱硫过程中的协同作用与机理[D].唐山:华北理工大学,2017. |
| ZHANG Shunyu.Synergistic effect and mechanism of steel slag composition in flue gas desulfurization[D].Tangshan:North China University of Science and Technology,2017. | |
| 3 | 魏建鹏.石灰石-石膏湿法脱硫系统节能降耗策略研究[C]//中国环境科学学会2021年科学技术年会——环境工程技术创新与应用分会场.天津:《环境工程》编辑部,2021. |
| 4 | 苏少龙,曲晓龙,钟读乐,等.工业烟气脱硫工艺进展[J].无机盐工业,2019,51(11):13-15,87. |
| SU Shaolong, QU Xiaolong, ZHONG Dule, et al.Progress of industrial flue gas desulfurization process[J].Inorganic Chemicals Industry,2019,51(11):13-15,87. | |
| 5 | 龙勇.高硫煤烟气氨法脱硫装置升级改造[J].氮肥与合成气,2021,49(11):14-16. |
| LONG Yong.Upgrading of high-sulfur coal flue gas ammonia desulfurization unit[J].Nitrogenous Fertilizer & Syngas,2021,49(11):14-16. | |
| 6 | 樊河雲,李瑛,赖立践,等.钢渣/碱式碳酸镁新型复合脱硫剂的性能研究[J].工业加热,2017,46(4):47-51. |
| FAN Heyun, LI Ying, LAI Lijian, et al.The desulfurization performance research of new-type composite desulphurizer of basic magnesium carbonate desulfurization agent with steel slag[J].Industrial Heating,2017,46(4):47-51. | |
| 7 | 张顺雨,贵永亮,宋春燕,等.钢渣烧结烟气脱硫现状[J].矿产综合利用,2018(1):1-5. |
| ZHANG Shunyu, GUI Yongliang, SONG Chunyan, et al.Present situation of sintering flue gas desulfurization with steel slag[J].Multipurpose Utilization of Mineral Resources,2018(1):1-5. | |
| 8 | 张国成,白晓光,邬虎林,等.钢渣脱硫剂用于湿法石灰石-石膏法脱硫工艺的试验研究[J].钢铁研究学报,2020,32(7):647-653. |
| ZHANG Guocheng, BAI Xiaoguang, WU Hulin, et al.Experimental study on desulfurization process of wet limestone-gypsum with steel slag desulfurizer[J].Journal of Iron and Steel Research,2020,32(7):647-653. | |
| 9 | 王高峰,王义忠,於珩,等.转炉钢渣用于烟气脱硫实践研究[C]//陕西省金属学会.第五届全国冶金渣固废回收及资源综合利用、节能减排高峰论坛.昆明:2020. |
| 10 | 王会刚,彭犇,岳昌盛,等.钢渣改性研究进展及展望[J].环境工程,2020,38(5):133-137,106. |
| WANG Huigang, PENG Ben, YUE Changsheng, et al.Research progress and prospect of steel slag modification[J].Environmental Engineering,2020,38(5):133-137,106. | |
| 11 | 丁希楼,郭应春,唐胜卫,等.废钢渣粉渣湿法脱硫工艺实验研究[J].环境工程,2009,27(3):99-102. |
| DING Xilou, GUO Yingchun, TANG Shengwei, et al.Experimental study on wet flue gas desulfurization with scrap slag powder residue[J].Environmental Engineering,2009,27(3):99-102. | |
| 12 | 孟子衡,王晨晔,王兴瑞,等.焦炉烟气钢渣湿法联合脱硫脱硝试验研究[J].洁净煤技术,2020,26(6):210-216. |
| MENG Ziheng, WANG Chenye, WANG Xingrui, et al.Experimental study on simultaneous desulfurization and denitrification by steelmaking slag for coke oven flue gas[J].Clean Coal Technology,2020,26(6):210-216. | |
| 13 | 徐露,范鼎东,夏能伟,等.钢渣微粉用于烧结烟气干法脱硫的试验研究[J].烧结球团,2015,40(3):48-52. |
| XU Lu, FAN Dingdong, XIA Nengwei, et al.Experimental study on dry sintering flue gas desulfurization with steel slag powder[J].Sintering and Pelletizing,2015,40(3):48-52. | |
| 14 | 邱伟,刘盛余,能子礼超,等.氢氧化钠-钢渣双碱法烧结烟气脱硫工艺[J].环境工程学报,2013,7(3):1095-1100. |
| QIU Wei, LIU Shengyu, NENG Zilichao, et al.Study of NaOH-steel slag double alkali sintering flue gas desulphurization process[J].Chinese Journal of Environmental Engineering,2013,7(3):1095-1100. | |
| 15 | 刘盛余,邱伟,汪雪婷,等.柠檬酸强化钢渣湿法烧结烟气脱硫及机理[J].环境工程学报,2015,9(3):1323-1328. |
| LIU Shengyu, QIU Wei, WANG Xueting, et al.Mechanism and process of steel slag agglomeration gas desulfurization enhanced by citric acid[J].Chinese Journal of Environmental Engineering,2015,9(3):1323-1328. | |
| 16 | 孟子衡.低温烟气钢渣联合脱硫脱硝过程强化工艺与机理研究[D].北京:中国科学院大学(中国科学院过程工程研究所),2020. |
| MENG Ziheng.Intensification technology and mechanism for simultaneous desulphurization and denitration from low-temperature flue gas by steel slag[D].Beijing:Institute of Process Engineering,Chinese Academy of Sciences,2020. | |
| 17 | 杨小白,韩云龙,李迎根,等.活性炭混合钢渣烧结烟气脱硫脱硝实验研究[J].过程工程学报,2019,19(2):440-446. |
| YANG Xiaobai, HAN Yunlong, LI Yinggen, et al.Experimental study on desulfurization and denitration of sintering flue gas by activated carbon mixed with steel slag[J].The Chinese Journal of Process Engineering,2019,19(2):440-446. | |
| 18 | 王彦斐.添加钢渣的水菱镁复合脱硫剂脱硫性能及实验研究[D].昆明:昆明理工大学,2019. |
| WANG Yanfei.Desulfurization performance and experimental study of hydromagnesite composite desulfurizer added with steel slag[D].Kunming:Kunming University of Science and Technology,2019. | |
| 19 | 陈捷.Na2CO3(CaCO3)/铁渣复合脱硫剂脱硫性能的研究[D].昆明:昆明理工大学,2016. |
| CHEN Jie.Study on desulfurization performance of Na2CO3(CaCO3)/iron slag composite desulfurizer[D].Kunming:Kunming University of Science and Technology,2016. | |
| 20 | 刘盛余,邱伟,吴萧,等.鼓泡塔中钢渣湿法烧结烟气脱硫过程及机理[J].应用基础与工程科学学报,2017,25(1):46-55. |
| LIU Shengyu, QIU Wei, WU Xiao, et al.Mechanism and process of sintering flue gas desulfurization by steel slag in bubbling tower[J].Journal of Basic Science and Engineering,2017,25(1):46-55. | |
| 21 | 张顺雨,贵永亮,袁宏涛,等.钢渣用于烧结烟气脱硫反应机理分析[J].铸造技术,2016,37(12):2650-2653. |
| ZHANG Shunyu, GUI Yongliang, YUAN Hongtao, et al.Mechanism analysis of desulfurization with steel slag for sintering flue gas[J].Foundry Technology,2016,37(12):2650-2653. | |
| 22 | LIU Xiaolong, YE Meng, WANG Xue, et al.Gas-phase and particle-phase PCDD/F congener distributions in the flue gas from an iron ore sintering plant[J].Journal of Environmental Sciences,2017,54: 239-245. |
| 23 | CHEN Shuhao, WANG Xinhua, HE Xiaofei, et al.Industrial application of desulfurization using low basicity refining slag in tire cord steel[J].Journal of Iron and Steel Research,International,2013,20(1):26-33. |
| 24 | 兰颖,马平.湿法烟气脱硫系统脱硫效率的影响因素分析[J].电力科学与工程,2013,29(7):58-63. |
| LAN Ying, MA Ping.Analysis of the factors of desulfurization efficiency in wet flue gas desulfurization system[J].Electric Power Science and Engineering,2013,29(7):58-63. | |
| 25 | 王丽秋,王小方,李会泉,等.焦炉烟气湿法钢渣联合脱硫脱硝工艺及机理研究[J].燕山大学学报,2016,40(4):348-354. |
| WANG Liqiu, WANG Xiaofang, LI Huiquan, et al.Research on technical method and mechanism of combined desulfurization and denitrification of flue gas from coking furnace with steel slag[J].Journal of Yanshan University,2016,40(4):348-354. |
| [1] | YANG En, SHEN Hongyan, LIU Youzhi. In situ modification of superfine magnesium hydroxide with silicon polyether [J]. Inorganic Chemicals Industry, 2024, 56(4): 42-49. |
| [2] | LI Qiaoyun, HUANG Xiuxing, WEI Wenye, CHEN Zhen. Study on adsorption of methylene blue by activated carbon with acid/alkali synergistically modified fly ash [J]. Inorganic Chemicals Industry, 2024, 56(3): 131-136. |
| [3] | JIN Shengshi, LIU Kaijie, LIU Qiuwen, ZHANG Yibo, YANG Xiangguang. Study on catalytic performance of phosphoric acid modified CeO2 nanorod supported Pt catalyst for propane combustion [J]. Inorganic Chemicals Industry, 2024, 56(1): 141-148. |
| [4] | ZHANG Conghua, YAN Wenbin, XIAO Jiajun, ZHAO Ke, PENG Shangquan, WEI Yuhong. Reductive leaching technology of manganese anode slag using tartaric acid as reducing agent optimized by RSM [J]. Inorganic Chemicals Industry, 2023, 55(9): 106-113. |
| [5] | LI Yang, ZANG Yihua, YUAN Biao, SHENG Chunguang. Modification of antifouling ceramic membrane and its application of oily wastewater treatment [J]. Inorganic Chemicals Industry, 2023, 55(9): 33-42. |
| [6] | FAN Fangfang, TONG Zhongkai, ZUO Weiyuan. Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar [J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115. |
| [7] | XU Li, ZHANG Qiang. Experimental study on properties of iron tailings powder cement-based materials [J]. Inorganic Chemicals Industry, 2023, 55(6): 116-123. |
| [8] | YUAN Enxian, LI Jinpeng, LI Qian, ZHOU Meixia, JIAN Panming. Preliminary study on cyclohexane catalytic oxidation over magnesium-doped tricobalt tetraoxide [J]. Inorganic Chemicals Industry, 2023, 55(6): 136-141. |
| [9] | HONG Meihua, GUO Zifeng, LIU Guanfeng, ZANG Jiazhong, YANG Keyu, YU Yonghua, ZHANG Dazhi, HUANG Shengjun. Progress and challenges of alkaline treatment for synthesis of hierarchical zeolites [J]. Inorganic Chemicals Industry, 2023, 55(6): 36-42. |
| [10] | DONG Linlin, GAO Huafeng. Study on preparation of steel slag phase change concrete modified by hydrofluoric acid solution [J]. Inorganic Chemicals Industry, 2023, 55(5): 109-114. |
| [11] | FENG Xiaoqian, ZHAO Yilin, ZHAO Yonghua, ZHANG Qijian, WANG Huan, MENG Qingrun. Recent progress of modified montmorillonited-based catalysts [J]. Inorganic Chemicals Industry, 2023, 55(5): 24-30. |
| [12] | ZHANG Chenhu, MA Yi, ZHU Shan, CHEN Peng, WANG Chengyong, LI Ziwen. Study on adsorption of heavy metal ions in mineral processing wastewater by chelating modified coal gangue [J]. Inorganic Chemicals Industry, 2023, 55(4): 97-103. |
| [13] | ZHOU Qiang, WU Bin, CHEN Kui, JI Lijun, WU Yanyang. Study on thermal decomposition kinetic mechanism and calcination process of phosphorus tailings [J]. Inorganic Chemicals Industry, 2023, 55(3): 47-54. |
| [14] | LIU Xueting, MAO Lingfeng, HU Yun, PENG Xi, FAN Xuemei, CHEN Yanlei, LIU Wenkui. Synergistic dispersion of SiO2 by dispersant and supershear [J]. Inorganic Chemicals Industry, 2023, 55(3): 71-77. |
| [15] | LI Peng, WANG Likun, MENG Qiuyan. Study on effect of α-hemihydrate gypsum on performance of cement mortar and its hydration mechanism [J]. Inorganic Chemicals Industry, 2023, 55(3): 98-103. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||