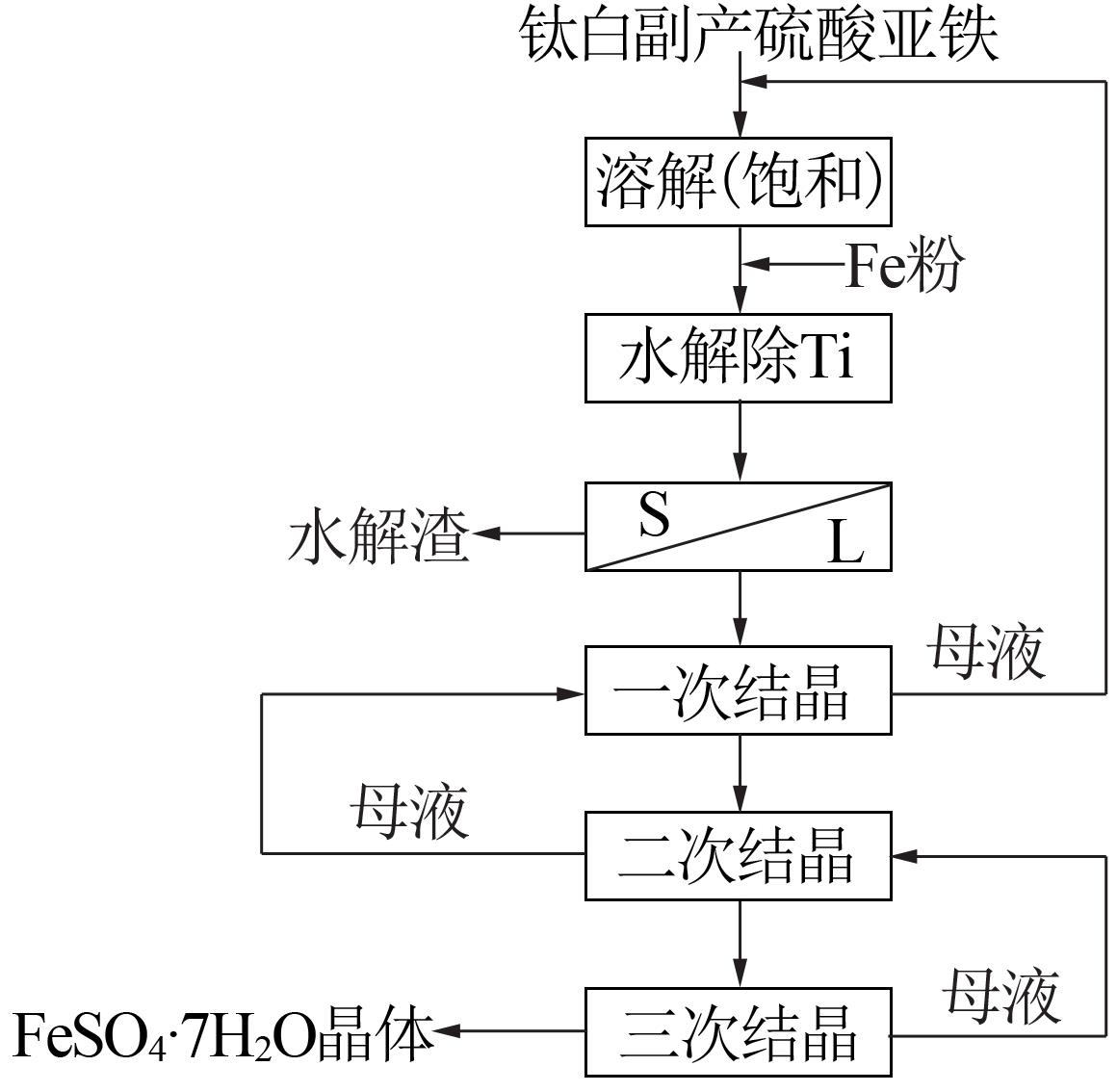
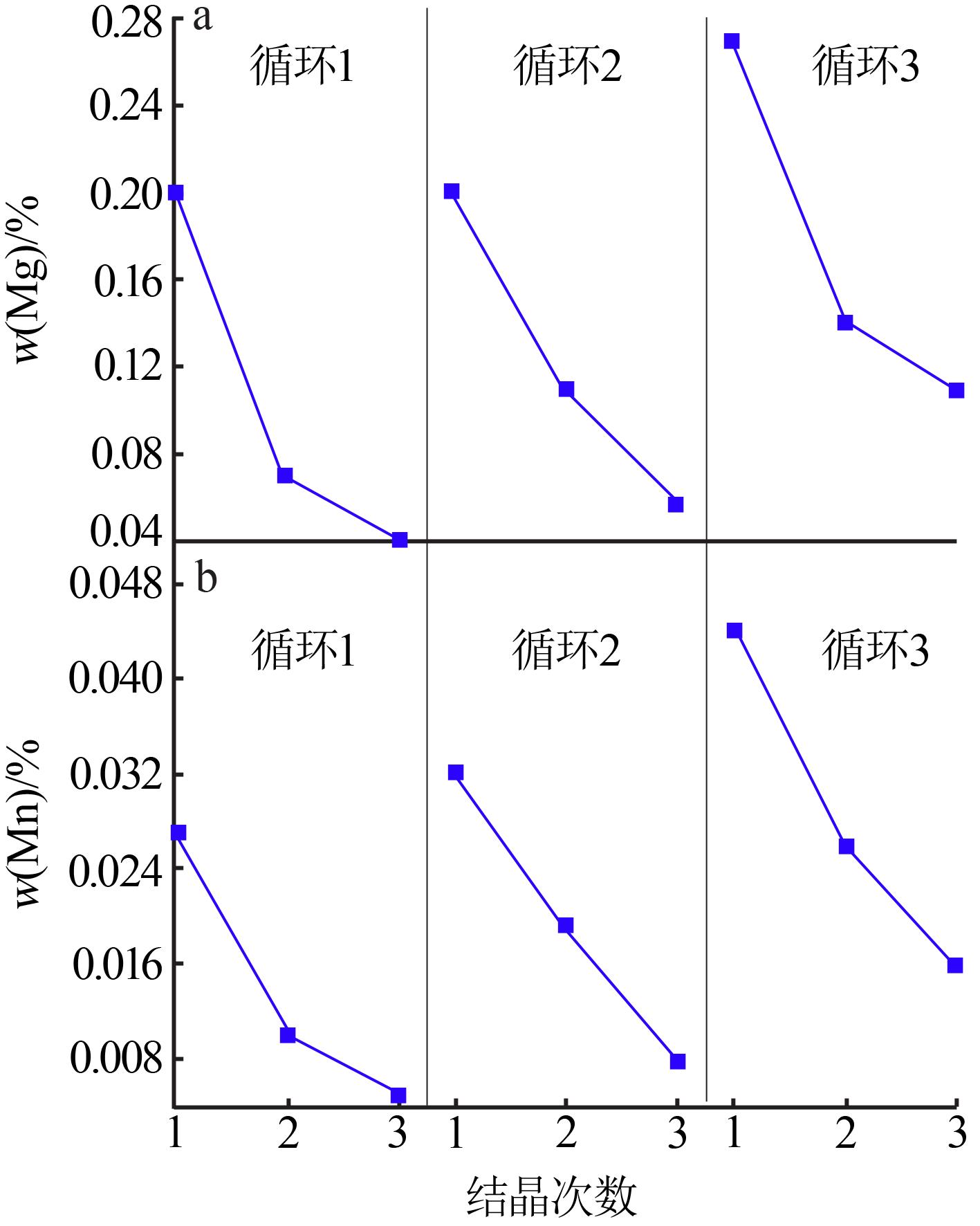
Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (7): 105-109.doi: 10.19964/j.issn.1006-4990.2021-0594
• Industrial Techniques • Previous Articles Next Articles
Study on impurity removal rule of ferrous sulfate from by-product of titanium dioxide by crystallization purification
ZHU Wanye1,2( ),TANG Ding1,2,CHI Heting1,2,LIAO Xianghui1,2,ZHUANG Rongchuan1,2,WANG Qiankun1,2,SHEN Qingfeng1,2
),TANG Ding1,2,CHI Heting1,2,LIAO Xianghui1,2,ZHUANG Rongchuan1,2,WANG Qiankun1,2,SHEN Qingfeng1,2
- 1.State Key Laboratory of Comprehensive Utilization of Low Grade Refractory Gold Ores, Shanghang 364200
2.Xiamen Zijin Mining and Metallurgical Technology Co. , Ltd.
-
Received:2021-09-30Online:2022-07-10Published:2022-07-14
CLC Number:
Cite this article
ZHU Wanye,TANG Ding,CHI Heting,LIAO Xianghui,ZHUANG Rongchuan,WANG Qiankun,SHEN Qingfeng. Study on impurity removal rule of ferrous sulfate from by-product of titanium dioxide by crystallization purification[J]. Inorganic Chemicals Industry, 2022, 54(7): 105-109.
share this article
Table 1
Composition analysis results of ferrous sulfate of titanium dioxide by-product and requirementsfor impurity content of FeSO4·7H2O crystal for battery"
| 项目 | w(Fe) | w(Ti) | w(Mg) | w(Mn) | w(Na) | w(Ca) | w(Al) | w(Co) | w(Cr) | w(Cu) | w(Ni) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 钛白副产硫酸亚铁 | 21.54 | 0.14 | 0.33 | 0.068 | <0.015 | <0.025 | <0.005 | <0.005 | <0.005 0 | <0.003 | <0.005 |
高纯七水硫酸亚铁晶体 (Q/WRN 0104—2016) | — | — | ≤0.025 | ≤0.05 | ≤0.015 | ≤0.025 | ≤0.005 | ≤0.005 | ≤0.005 | ≤0.003 | ≤0.005 |
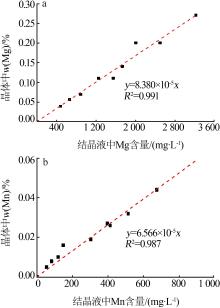
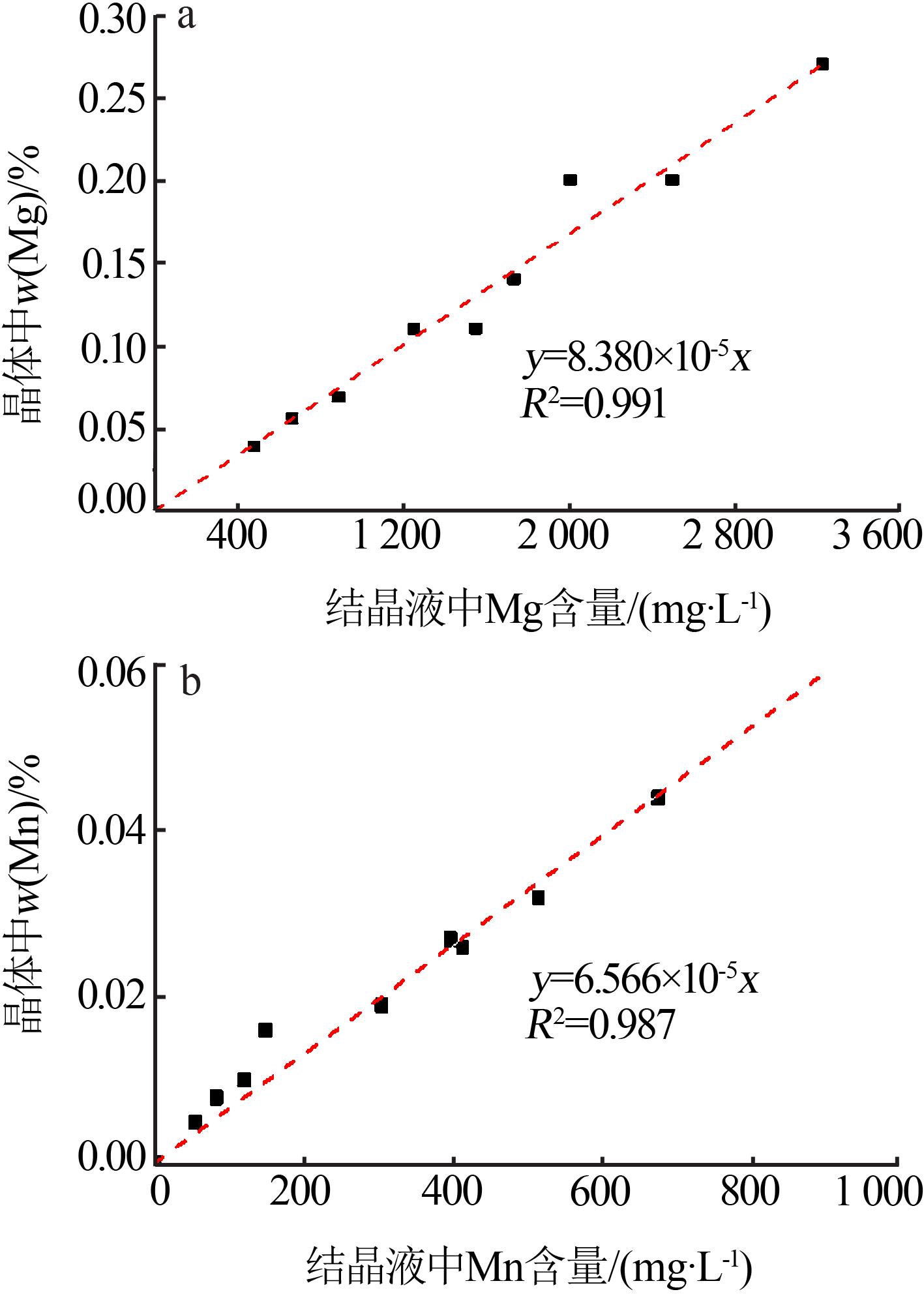
Table 2
Analysis results of element content in solution before and after Ti removal by hydrolysis with different pH"
| 样品 | 体积/ mL | pH | Fe粉用量/g | ρ(Fe)/ (mg?L-1) | ρ(Ti)/ (mg?L-1) | ρ(Mg)/ (mg?L-1) | ρ(Mn)/ (mg?L-1) | ρ(Na)/ (mg?L-1) | Ti 去除率/% | Fe 损失率/% |
|---|---|---|---|---|---|---|---|---|---|---|
| 原液 | 1 100 | 2.3 | — | 95 500 | 875.00 | 1 655 | 362.0 | 11.4 | — | — |
| 除Ti后液1 | 940 | 3.0 | 600 | 108 700 | 150.00 | 1 805 | 382.0 | 12.1 | 85.35 | 2.73 |
| 除Ti后液2 | 930 | 4.0 | 750 | 108 500 | 0.35 | 2 005 | 396.5 | 12.8 | 99.96 | 3.95 |
| 除Ti后液3 | 915 | 5.0 | 1 500 | 101 500 | 0.35 | 2 012 | 397.0 | 13.1 | 99.96 | 11.59 |
| 1 | 纪利春.硫酸法钛白副产物绿矾的资源化利用现状[J].无机盐工业,2020,52(5):11-17. |
| JI Lichun.Present status of resource utilization of by-product green vitriol from titanium dioxide production by sulfuric acid method[J].Inorganic Chemicals Industry,2020,52(5):11-17. | |
| 2 | 谢全模,胡文方,张莉,等.废渣绿矾精制高纯度硫酸亚铁的研究[J].河南化工,2007,24(11):33-35. |
| XIE Quanmo, HU Wenfang, ZHANG Li,et al.Research on the refining ferrous sulfate from waste copperas[J].Henan Chemical Industry,2007,24(11):33-35. | |
| 3 | 唐聪,陈泉源,吕璠璠,等.三维电极电Fenton氧化法处理染料废水[J].化工环保,2019,39(1):28-33. |
| TANG Cong, CHEN Quanyuan, Fanfan LÜ,et al.Treatment of dye wastewater by electro-Fenton oxidation process with three-dimensional electrode[J].Environmental Protection of Chemical Industry,2019,39(1):28-33. | |
| 4 | 谢四才.钛白粉厂硫酸亚铁资源综合利用实验研究[J].广东化工,2012,39(2):128-129. |
| XIE Sicai.An experimental study on comprehensive utilization of resources of titanium dioxide factory scrap ferrous sulfate[J]. | |
| Guangdong Chemical Industry,2012,39(2):128-129. | |
| 5 | 罗道成,易平贵,刘俊峰.硫铁矿烧渣综合利用研究进展[J].工业安全与环保,2003,29(4):10-12. |
| LUO Daocheng, YI Pinggui, LIU Junfeng.Study and development on the comprehensive utilization of iron pyrite cinder[J].Industrial Safety and Dust Control,2003,29(4):10-12. | |
| 6 | 钱有军,裴晓东,骆艳华,等.钛白副产物硫酸亚铁净化液制备高品质氧化铁红试验[J].金属矿山,2021(7):211-215. |
| QIAN Youjun, PEI Xiaodong, LUO Yanhua,et al.Preparation of high quality iron oxide red by purification solution of titanium by-products ferrous sulfate[J].Metal Mine,2021(7):211-215. | |
| 7 | 吴健春,路瑞芳.钛白副产废酸和七水硫酸亚铁制备一水硫酸亚铁的研究[J].无机盐工业,2018,50(6):75-77. |
| WU Jianchun, LU Ruifang.Study on preparation of FeSO4·H2O from titanium white waste acid and FeSO4·7H2O[J].Inorganic Che- | |
| Industry micals,2018,50(6):75-77. | |
| 8 | YANG Shoufeng, ZAVALIJ P Y, WHITTINGHAM M S.Hydrothermal synthesis of lithium iron phosphate cathodes[J].Electrochemistry Communications,2001,3(9):505-508. |
| 9 | 谷和云,李昇,李二锐,等.镁离子掺杂磷酸铁锂的制备及其电化学性能[J].无机盐工业,2016,48(1):64-67. |
| GU Heyun, LI Sheng, LI Errui,et al.Synthesis and electrochemical performance of magnesium ion doped lithium iron phosphate[J].Inorganic Chemicals Industry,2016,48(1):64-67. | |
| 10 | 冯颖,刘凯,霍涛涛,等.镁掺杂的非化学计量比磷酸铁锂的制备及性能[J].化工进展,2017,36(8):3006-3012. |
| FENG Ying, LIU Kai, HUO Taotao,et al.Synthesis and performance of Mg-doped non-stoichiometric lithium iron phosphate[J].Chemical Industry and Engineering Progress,2017,36(8):3006- | |
| 3012. | |
| 11 | XU Bo, QIAN Danna, WANG Ziying,et al.Recent progress in cat- |
| hode materials research for advanced lithium ion batteries[J].Materials Science and Engineering:R:Reports,2012,73(5/6):51-65. | |
| 12 | 李化全,张华军,郭传华.利用钛白副产硫酸亚铁制备正极材料磷酸铁锂的研究[J].山东化工,2018,47(4):27-28,34. |
| LI Huaquan, ZHANG Huajun, GUO Chuanhua.A study on lithium | |
| iron phosphate was prepared by using titanium dioxide as a byproduct of ferrous sulfate[J].Shandong Chemical Industry,2018,47(4):27-28,34. | |
| 13 | 郭举.钛白渣提纯制备电池级硫酸亚铁工艺技术研究[J].无机盐工业,2019,51(8):48-51. |
| GUO Ju.Study on preparation of battery-grade ferrous sulfate by purification of titanium white slag[J].Inorganic Chemicals Industry,2019,51(8):48-51. | |
| 14 | 张克宇,吴鉴,高腾飞,等.结晶法提纯钛白副产硫酸亚铁的数学模型与实验研究[J].高校化学工程学报,2016,30(2):371-377. |
| ZHANG Keyu, WU Jian, GAO Tengfei,et al.Simulation and experimental studies on purification of ferrous sulfate from titanium dioxide byproducts by crystallization[J].Journal of Chemical Engineering of Chinese Universities,2016,30(2):371-377. | |
| 15 | 刘世琦.一种电池级七水硫酸亚铁晶体的制备方法:中国,105293588[P].2007-03-22. |
| LIU Shiqi.A preparation method of battery grade ferrous sulfate heptahydrate crystal:CN,105293588[P].2007-03-22. | |
| 16 | 张克宇,吴鉴,周朝金,等.结晶法提纯钛白副产硫酸亚铁[J].有色金属工程,2017,7(2):10-15. |
| ZHANG Keyu, WU Jian, ZHOU Chaojin,et al.Purification of ferrous sulfate byproduct from titanium dioxide by crystallization process[J].Nonferrous Metals Engineering,2017,7(2):10-15. | |
| 17 | 舒均杰,田伟军.由钛白废酸和硫铁矿烧渣制备饲料级硫酸亚铁[J].无机盐工业,2009,41(2):50-52. |
| SHU Junjie, TIAN Weijun.Preparation of feed-grade ferrous sulfate by waste acid from titanium dioxide production and pyrite cinder[J].Inorganic Chemicals Industry,2009,41(2):50-52. | |
| 18 | 李建文.硫酸亚铁干燥条件及干燥特性的研究[J].饲料工业,1996,17(6):10-12. |
| LI Jianwen.Study on drying conditions and drying characteristics of ferrous sulfate [J].Feed Industry,1996,17(6):10-12. |
| [1] | LIU Jie, SHI Xueru, WEI Shubing, CAO Xinxin. Artificial interface layer construction and sodium storage performance of P2-type layered oxide cathode [J]. Inorganic Chemicals Industry, 2024, 56(3): 39-44. |
| [2] | ZHOU Huang, HU Xiaoping, REN Wen, CAO Xinxin. Preparation and sodium storage properties of sulfur-doped Na3(VOPO4)2F cathode materials [J]. Inorganic Chemicals Industry, 2024, 56(2): 30-37. |
| [3] | WEI Tianshun, JI Lijun, SHENG Yong, CHEN Kui, WU Yanyang, WU Bin. Study on fractional crystallization process of ammonium sulfate and sodium sulfate in high salt wastewater [J]. Inorganic Chemicals Industry, 2024, 56(1): 102-106. |
| [4] | CHEN Xu, YANG Gang, LI Haitao. Study on preparation of multistage pore nano-SAPO-34 molecular sieves and its methanol to olefin performance [J]. Inorganic Chemicals Industry, 2024, 56(1): 134-140. |
| [5] | GAN Shunpeng, DING Ding, SUN Chenggao, JIANG Shipeng, GUAN Junfang. Study on cold decomposition crystallization process of underground primary high-sodium carnallite ore [J]. Inorganic Chemicals Industry, 2024, 56(1): 67-72. |
| [6] | XIONG Xiaoyun, CAO Gengzhen, DU Xuemin, WANG Jiujiang, HU Qingxun. Preparation of macroporous in situ crystallized FCC catalyst by carbon black template method [J]. Inorganic Chemicals Industry, 2023, 55(9): 134-139. |
| [7] | LI Yan, MA Zhen, SONG Xingfu. New development advance and industrial development proposals of chloride-type salt lake potassium resources in Qinghai [J]. Inorganic Chemicals Industry, 2023, 55(8): 84-90. |
| [8] | WANG Song, WANG Jiawei, GOU Bibo, YANG Pan, HE Yue, YANG Chunyuan, WANG Haifeng. Study on distribution law of magnesium in leaching solution of rhodochrosite by complex salt crystallization method [J]. Inorganic Chemicals Industry, 2023, 55(4): 65-71. |
| [9] | WANG Lijuan, YAN Kezhou, GUO Zhiqiang, ZHAO Zhonghe, GUO Yanxia, CHENG Fangqin. Preparation of poly-aluminum chloride from acid leaching liquor of red mud-coal gangue activated by sodium salt [J]. Inorganic Chemicals Industry, 2023, 55(4): 76-83. |
| [10] | WEI Kang, ZHANG Hao, GAN Shunpeng. Research on mechanical drive enhancement crystallization process for titanium gypsum [J]. Inorganic Chemicals Industry, 2023, 55(11): 78-85. |
| [11] | PENG Jiaoyu, TAN Yuqin, YANG Keli, DONG Yaping, ZHANG Bo, LI Wu. Study on crystallization mechanism and kinetics of macallisterite synthesized with bischofite from salt lake [J]. Inorganic Chemicals Industry, 2023, 55(10): 56-62. |
| [12] | WANG Xiaoqian,SONG Huiping,XUE Fangbin. Research progress on preparation and application of inorganic salt whiskers [J]. Inorganic Chemicals Industry, 2022, 54(8): 20-32. |
| [13] | HE Lei,ZHU Ganyu,ZHENG Guangming,WU Wenfen,ZhANG Jianbo,LI Fang,LI Huiquan,CHEN Wen. Study on crystallization process and mechanism of phosphogypsum in wet process phosphoric acid system [J]. Inorganic Chemicals Industry, 2022, 54(7): 110-116. |
| [14] | ZHOU Zhixin,WANG Bo,XU Dehua,YANG Xiushan,ZHANG Zhiye. Study on antisolvent crystallization process in calcium nitrate-phosphoric acid-water-isopropanol system [J]. Inorganic Chemicals Industry, 2022, 54(7): 55-60. |
| [15] | DONG Peng,ZHOU Yingjie,HOU Minjie,YANG Dongrong,DAI Yongnian,LIANG Feng. Research progress on Na3V2(PO4)3 cathode materials for sodium ion batteries [J]. Inorganic Chemicals Industry, 2022, 54(5): 1-10. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||