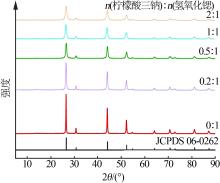
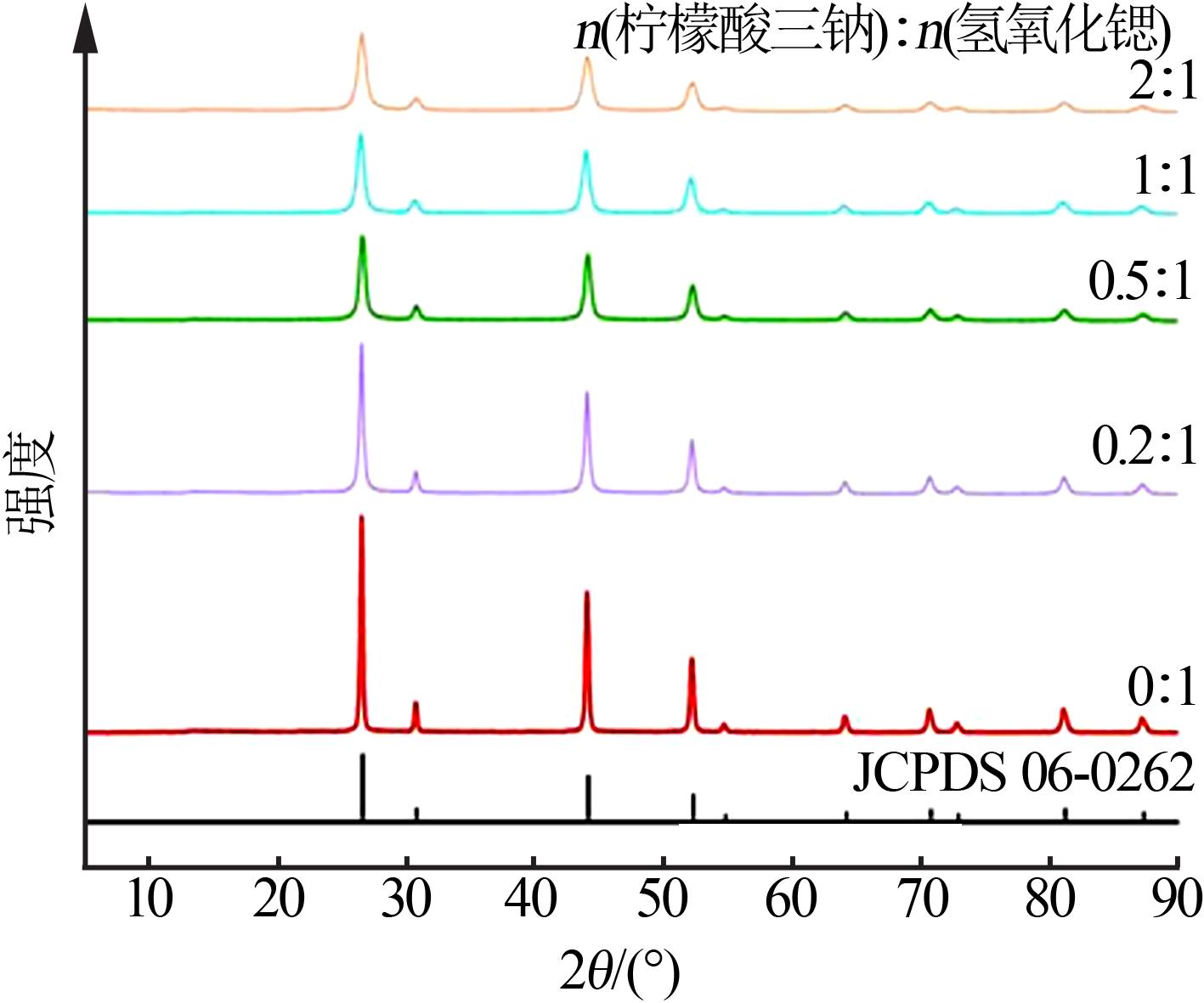
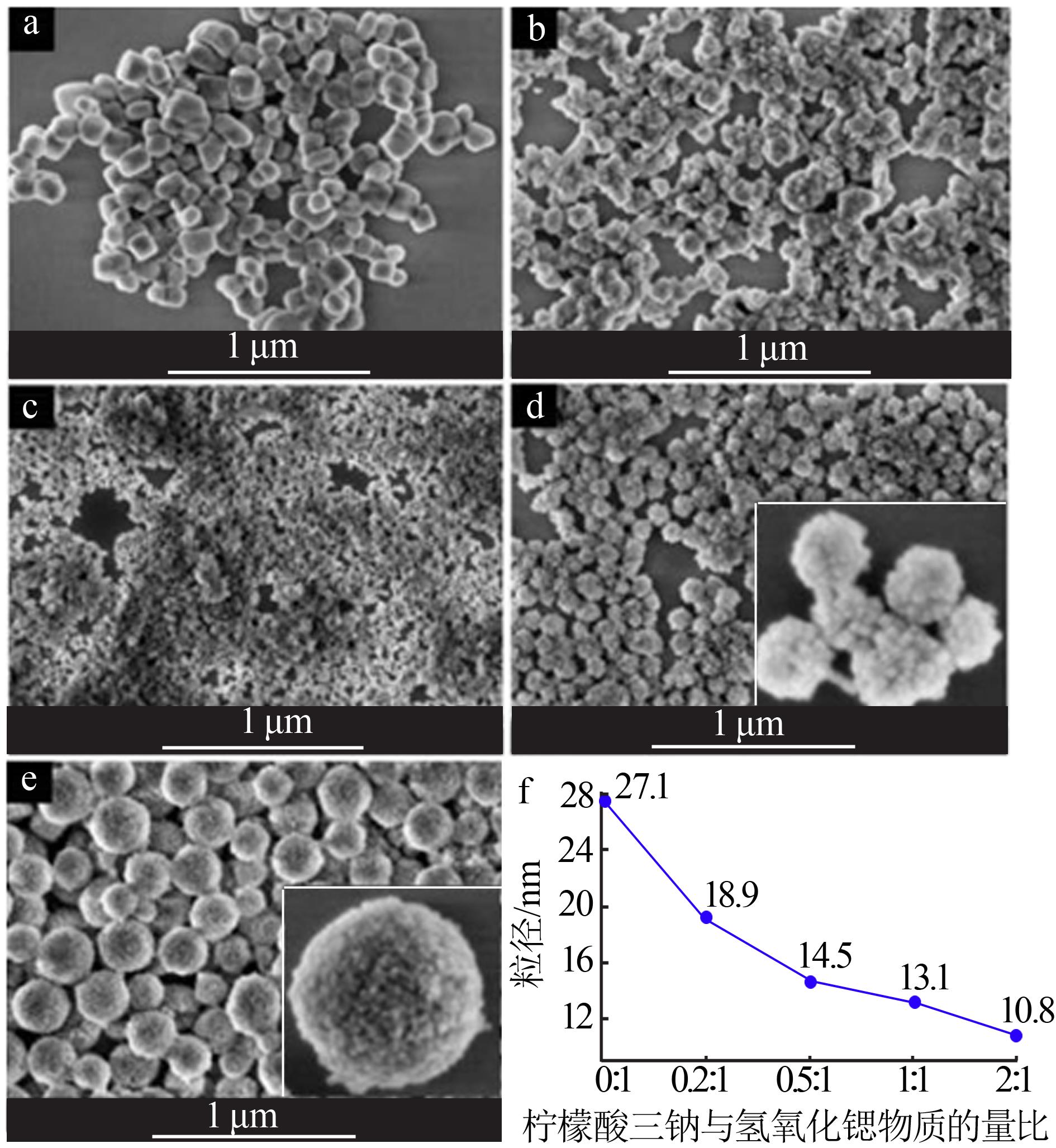
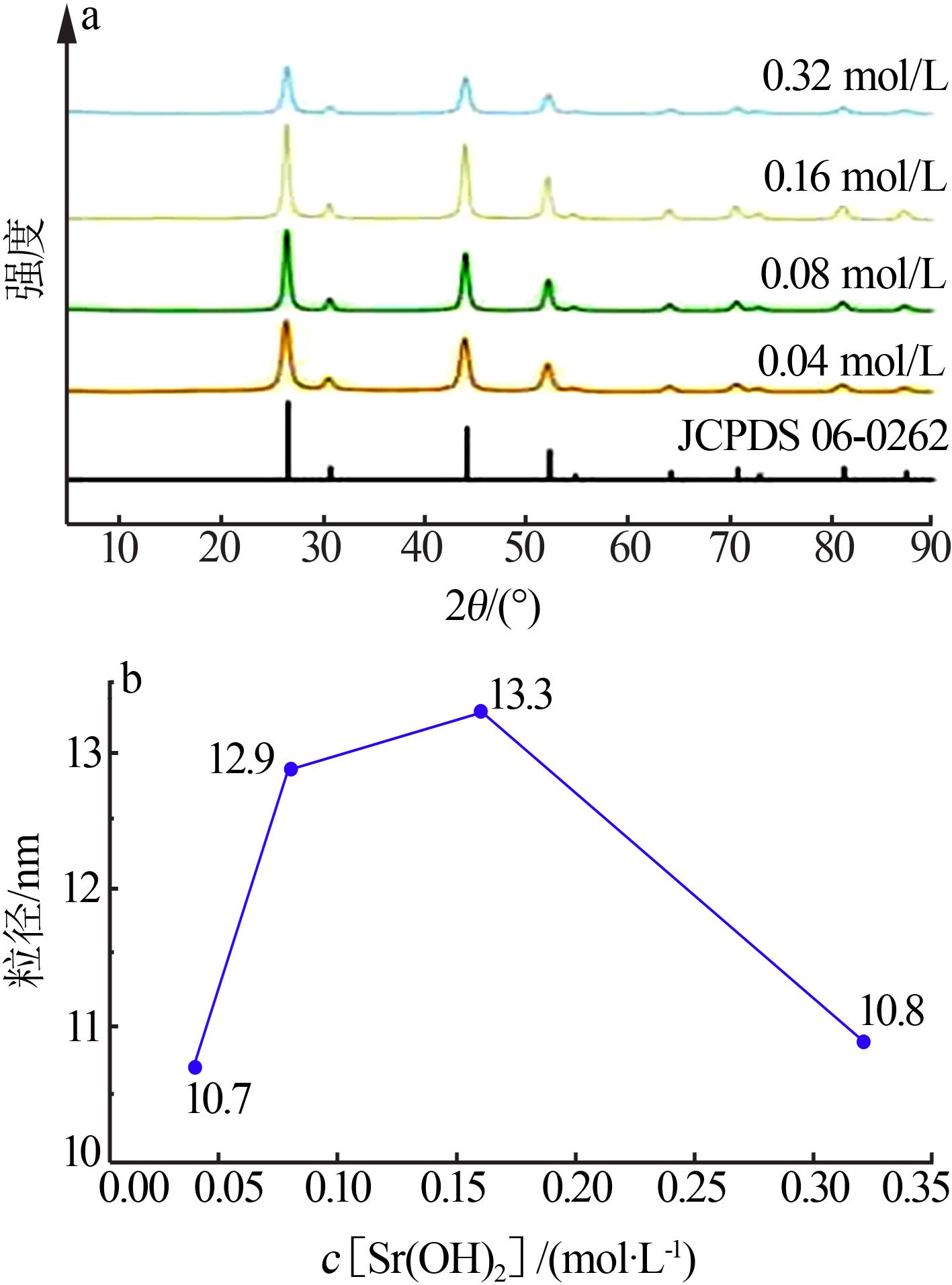
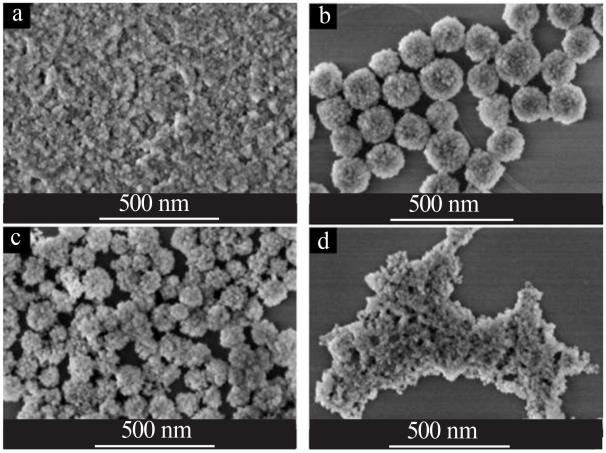
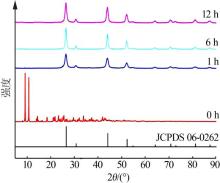
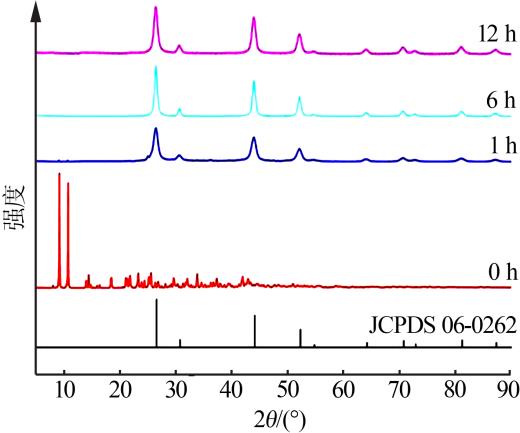
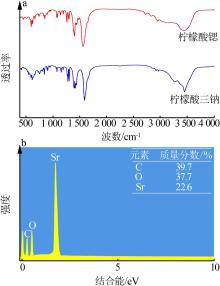
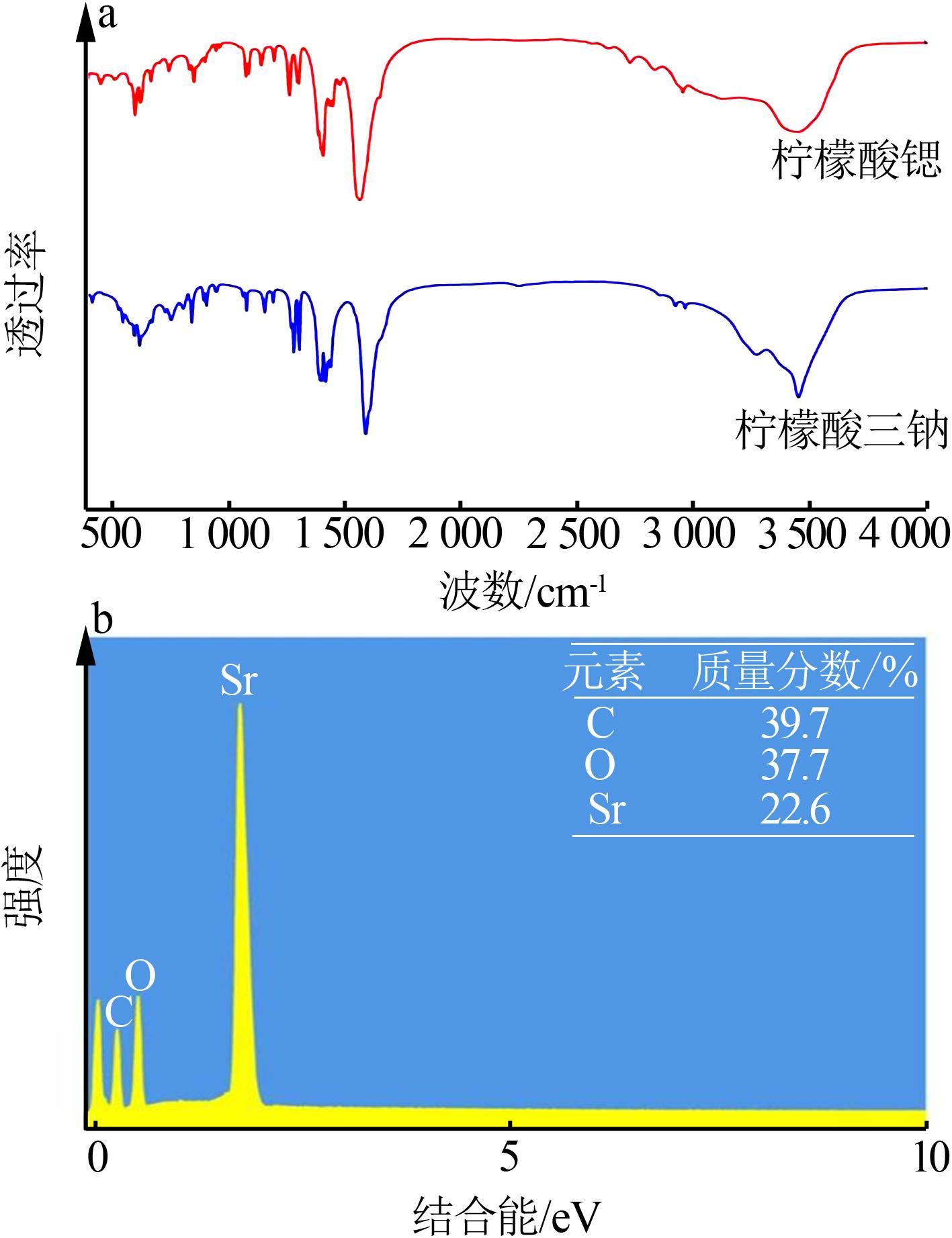
| [1] |
DI Lu, WANG Weiguo, CHEN Juexian, WU Chuanshu.
Study on preparation of transition metal-supported Silicalite-1 zeolite catalyst and its catalytic performance for furfural hydrogenation
[J]. Inorganic Chemicals Industry, 2024, 56(4): 125-132.
|
| [2] |
CHEN Zhangxu, FU Minglian, ZHU Danchen, ZHENG Bingyun.
Preparation of carbon/graphite carbon nitride composites and their methylene blue removal performance
[J]. Inorganic Chemicals Industry, 2023, 55(3): 134-139.
|
| [3] |
JIANG Demin, LI Shunmei, Li Qingqing, Chen Yuxin, LIU Daijun.
Hydrothermal synthesis of basic magnesium sulfate whiskers from magnesium hydroxide and magnesium sulfate heptahydrate
[J]. Inorganic Chemicals Industry, 2023, 55(12): 74-81.
|
| [4] |
LU Yang,WANG Jing,HE Lijie,WANG Fudong,ZHANG Jiaming.
Research progress on preparation of magnesium aluminate spinel powders and it in field of luminescent material
[J]. Inorganic Chemicals Industry, 2022, 54(9): 39-46.
|
| [5] |
LU Zheng,CHEN Kunfeng,XUE Dongfeng.
Study on large-scale preparation and electrochemical properties of high thermal stabilized α-Fe2O3
[J]. Inorganic Chemicals Industry, 2022, 54(3): 45-50.
|
| [6] |
PEI Yinchang,MO Shengpeng,XIE Qinglin,CHEN Nanchun.
Study on mechanism of zeolite A synthesized from stellerite zeolite by two-step hydrothermal method
[J]. Inorganic Chemicals Industry, 2022, 54(11): 59-64.
|
| [7] |
QI Yuanhao,WU Jinxiu,LIU Zhaogang,HU Yanhong,FENG Fushan,LI Jianfei,WANG Xin,LIU Conglin.
Preparation and characterization of anhydrous calcium sulfate whisker
[J]. Inorganic Chemicals Industry, 2022, 54(10): 109-115.
|
| [8] |
Zhang Shangqiang,Sun Guanhua,Sun Yanmin,Zhu Jinjian,Nan Jun,Xiao Han,Zhang Jingcheng,Song Guoliang.
Study on preparation of CNTs-Al2O3 supported Pd catalyst and its performance
[J]. Inorganic Chemicals Industry, 2021, 53(6): 194-198.
|
| [9] |
ZHU Danchen,LI Mingjie,CHEN Zhangxu,LIN Qian.
Study on hydrothermal synthesis of MnO2 and its decolorization performance on dyes
[J]. Inorganic Chemicals Industry, 2021, 53(11): 60-65.
|
| [10] |
Li Xiangguo,Guo Miao,Yao Huimin,Lü Jing.
Research on hydrothermal synthesis and photoluminescence mechanism of rhombus CeO2
[J]. Inorganic Chemicals Industry, 2020, 52(6): 30-35.
|
| [11] |
Guo Ju,Jia Shuangzhu.
Study on the one-step hydrothermal synthesis of LiFePO4 and its properties
[J]. Inorganic Chemicals Industry, 2020, 52(6): 36-40.
|
| [12] |
Liu Ying.
Synthesis of magnesium ferrite photocatalyst and degradation activity of various dyes
[J]. Inorganic Chemicals Industry, 2020, 52(11): 103-107.
|
| [13] |
Huang Zhenxu,He Huanhuan,Jia Panpan,Chen Tiwei,Wei Shiqian.
Synthesis of graphene by hydrothermal method and its electrocatalytic property on ascorbic acid
[J]. Inorganic Chemicals Industry, 2020, 52(11): 29-32.
|
| [14] |
Chen Ping,Tian Yu,Hu Cheng.
Effect of type and concentration of salt solution on synthesis of α-semi-water desulfurization gypsum
[J]. Inorganic Chemicals Industry, 2020, 52(10): 130-134.
|
| [15] |
Wang Donghua,Fu Xin.
Preparation and properties of iron oxide nanomaterials with different morphologies
[J]. Inorganic Chemicals Industry, 2019, 51(9): 21-23.
|
 ),ZHAO Yuxiang1,2,LI Bo1,2,ZOU Xingwu1,2,WANG Shuxuan1,2(
),ZHAO Yuxiang1,2,LI Bo1,2,ZOU Xingwu1,2,WANG Shuxuan1,2( )
)