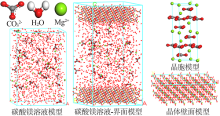
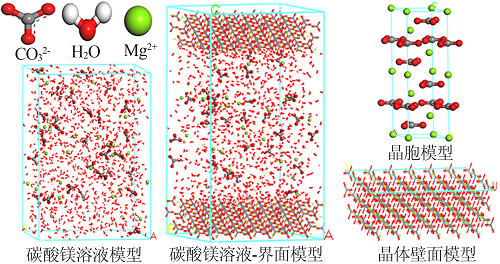
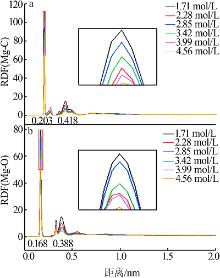
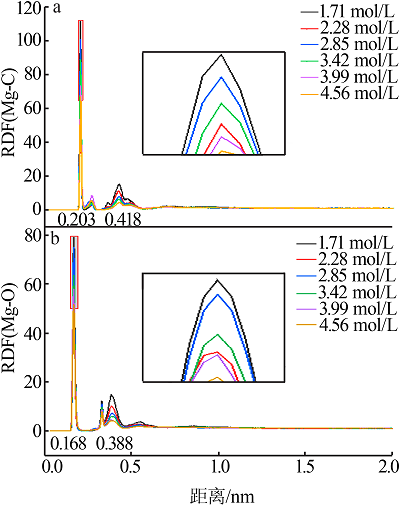
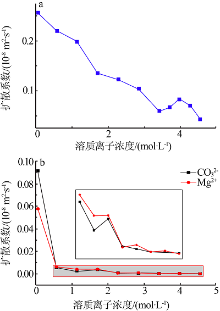
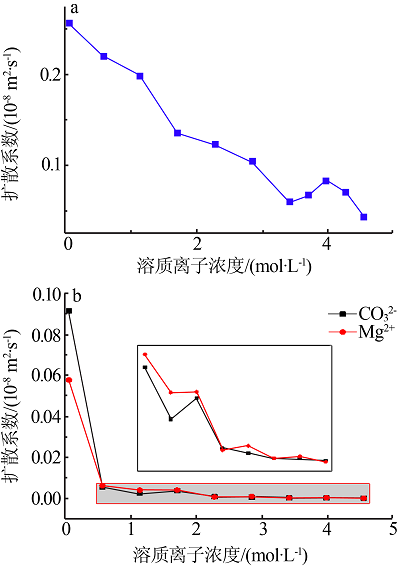
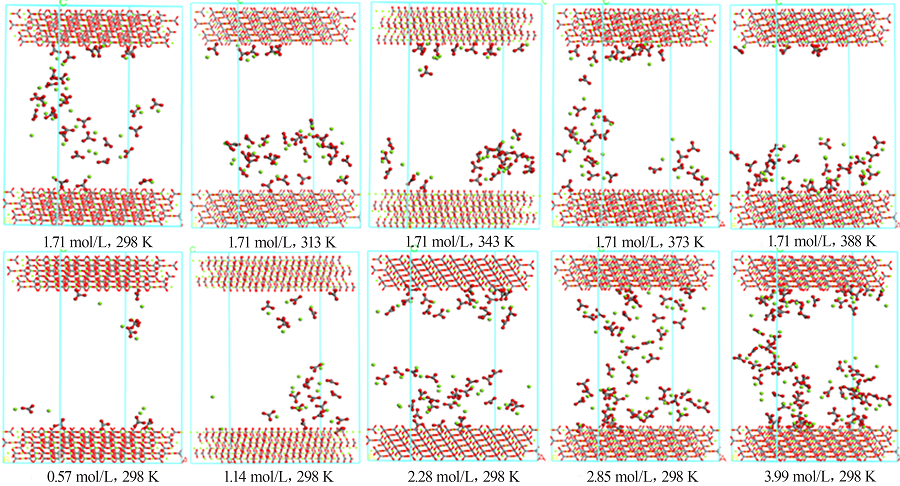
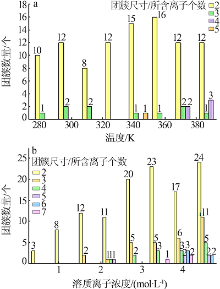
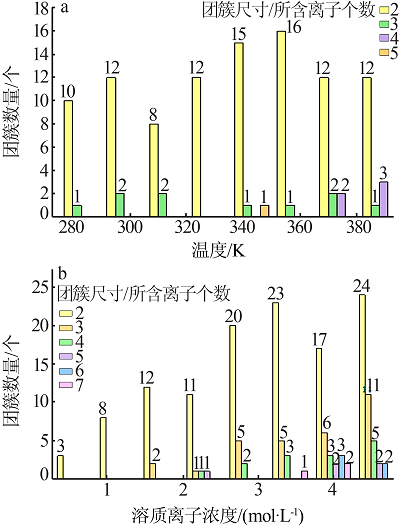
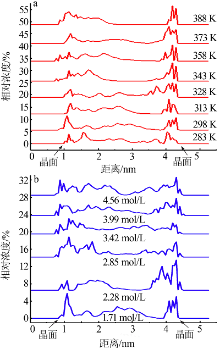
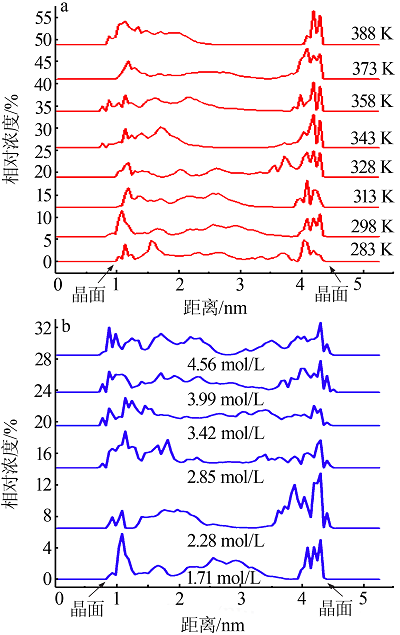
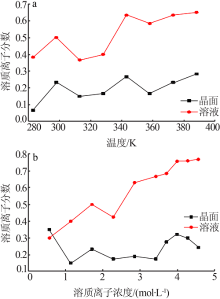
| [1] |
王英伟. 超细无水碳酸镁粉体的制备[D]. 北京:北京化工大学, 2017.
|
| [2] |
NI S, LI T, YANG X L. Hydrothermal synjournal of MgCO3 and its op-tical properties[J]. Journal of Alloys Compound, 2011, 509:7874-7876.
doi: 10.1016/j.jallcom.2011.04.064
|
| [3] |
XU J, YAN C, ZHANG F F, et al. Testing the cation-hydration effect on the crystallization of Ca-Mg-CO3 systems[J]. Proceedings of the National Academy of Sciences, 2013, 110:17750-17755.
doi: 10.1073/pnas.1307612110
|
| [4] |
TIAN P, YE J W, XU N, et al. A magnesium carbonate recyclable template to synthesize micro hollow structures at a large scale[J]. Chemical Communications, 2011, 47:12008-12010.
doi: 10.1039/c1cc15186j
|
| [5] |
CUI W W, LI P, WANG Z M, et al. Adsorption study of selenium ions from aqueous solutions using MgO nanosheets synthesized by ultra-sonic method[J]. Journal of Hazardous Materials, 2018, 341:268-276.
doi: 10.1016/j.jhazmat.2017.07.073
|
| [6] |
REZAEI F, MOUSSAVI G, BAKHTIARI A R, et al. Toluene removal from waste air stream by the catalytic ozonation process with MgO/GAC composite as catalyst[J]. Journal of Hazardous Materials, 2016, 306:348-358.
doi: 10.1016/j.jhazmat.2015.11.026
|
| [7] |
MIRTALEBI S S, ALMASI H, KHALEDABAD M A. Physical,mor-phological,antimicrobial and release properties of novel MgO-bac-terial cellulose nanohybrids prepared by in-situ and ex-situ metho-ds[J]. International Journal of Biological Macromolecules, 2019, 128:848-857.
doi: 10.1016/j.ijbiomac.2019.02.007
|
| [8] |
张翠钰, 方莉, 廖洪强, 等. 三水碳酸镁在氢氧化镁-二氧化碳-水体系中的沉淀结晶[J]. 无机盐工业, 2018, 50(6):36-41.
|
| [9] |
王晶瑶, 郭宏飞, 吴耀, 等. 苦卤制备碱式碳酸镁及分离钠、钾盐工艺研究[J]. 高校化学工程学报, 2020, 34(2):383-392.
|
| [10] |
SANDENGEN K, JOSANG L O, KAASA B. Simple method for synjournal of magnesite(MgCO3)[J]. Industrial & Engineering Chemistry Research, 2008, 47(4):1002-1004.
doi: 10.1021/ie0706360
|
| [11] |
KORNPROBST T, PLANK J. Synjournal and properties of magnesium carbonate xerogels and aerogels[J]. Journal of Non-Crystalline So-lids, 2013, 361:100-105.
|
| [12] |
高震. 特殊形貌无水碳酸镁及氧化镁粉体制备和性能表征[D]. 大连:大连交通大学, 2011.
|
| [13] |
SWANSON E J, FRICKER K J, SUN M, et al. Directed precipita-tion of hydrated and anhydrous magnesium carbonates for carbon storage[J]. Physical Chemistry Chemical Physics, 2014, 16(42):23440-23450.
doi: 10.1039/C4CP03491K
|
| [14] |
杨静, 陈明洋, 许史杰, 等. 无机盐晶体形貌调控研究进展[J]. 无机盐工业, 2018, 50(8):11-15.
|
| [15] |
李继翔, 郝婷婷, 兰忠, 等. 射流循环DTB结晶器内的CFD模拟[J]. 化工学报, 2015, 66(9):3383-3390.
|
| [16] |
YAN X, LI Z, ZOU C, et al. Renucleation and sequential growth of ZnO complex nano/microstructure:From nano/microrod to ball-sh-aped cluster[J]. The Journal of Physical Chemistry C, 2009, 114(3):1436-1443.
doi: 10.1021/jp908101z
|
| [17] |
徐威. 冷凝成核微观过程与界面结构效应影响机制[D]. 大连:大连理工大学, 2015.
|
| [18] |
王雪. 颗粒聚集生长过程的动力学模拟[D]. 北京:中国科学院大学(中国科学院过程工程研究所), 2018.
|
| [19] |
LANARO G, PATEY G N. Birth of NaCl crystals:Insights from mo-lecular simulations[J]. The Journal of Physical Chemistry B, 2016, 120(34):9076-9087.
doi: 10.1021/acs.jpcb.6b05291
|
| [20] |
CHAKRABORTY D, PATEY G N. How crystals nucleate and grow in aqueous NaCl solution[J]. The Journal of Physical Chemistry Letters, 2013, 4(4):573-578.
doi: 10.1021/jz302065w
|
| [21] |
窦翔宇. 反应-扩散调控材料成核过程的分子动力学模拟[D]. 北京:中国科学院大学(中国科学院过程工程研究所), 2020.
|
| [22] |
KADOTA K, SHIRAKAWA Y, WADA M, et al. Influence of clus-ters on the crystal surface of NaCl at initial growth stage investiga-ted by molecular dynamics simulations[J]. Journal of Molecular Liquids, 2012, 166(1):31-39.
doi: 10.1016/j.molliq.2011.11.009
|
| [23] |
YAMANAKA S, SHIMOSAKA A, SHIRAKAWA Y, et al. Molec-ular dynamics simulations of the formation for NaCl cluster at the interface between the supersaturated solution and the substrate[J]. Journal of Nanoparticle Research, 2010, 12(3):831-839.
doi: 10.1007/s11051-009-9713-z
|
| [24] |
KADOTA K, FURUKAWA R, SHIRAKAWA Y, et al. Effect of sur-face properties of calcium carbonate on aggregation process inves-tigated by molecular dynamics simulation[J]. Journal of Materials Science, 2014, 49(4):1724-1733.
doi: 10.1007/s10853-013-7859-7
|
| [25] |
柴汝宽, 刘月田, 杨莉, 等. 方解石表面结构影响水分子吸附的微观机理[J]. 哈尔滨工业大学学报, 2020, 52(4):170-179.
|
| [26] |
柴林涛, 乔秀臣, 秦东, 等. 结晶硫酸铝脱水过程中晶型与形貌的转变[J]. 无机盐工业, 2016, 48(11):17-20.
|
| [27] |
SHI W Y, XU W, CANG H, et al. Design and synjournal of biode-gradable antiscalant based on MD simulation of antiscale mecha-nism:A case of itaconic acid-epoxysuccinate copolymer[J]. Com-putational Materials Science, 2017, 136:118-125.
|
 ),QIANG Weili,LIAN Shijun,LAN Zhong(
),QIANG Weili,LIAN Shijun,LAN Zhong( )
)