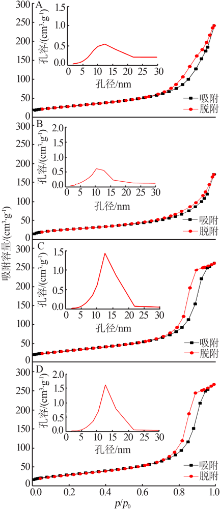
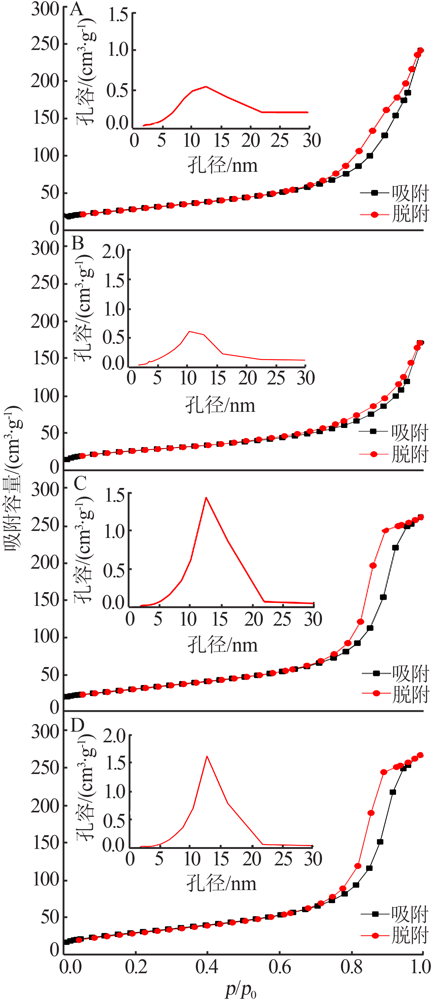
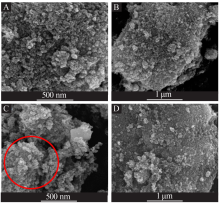
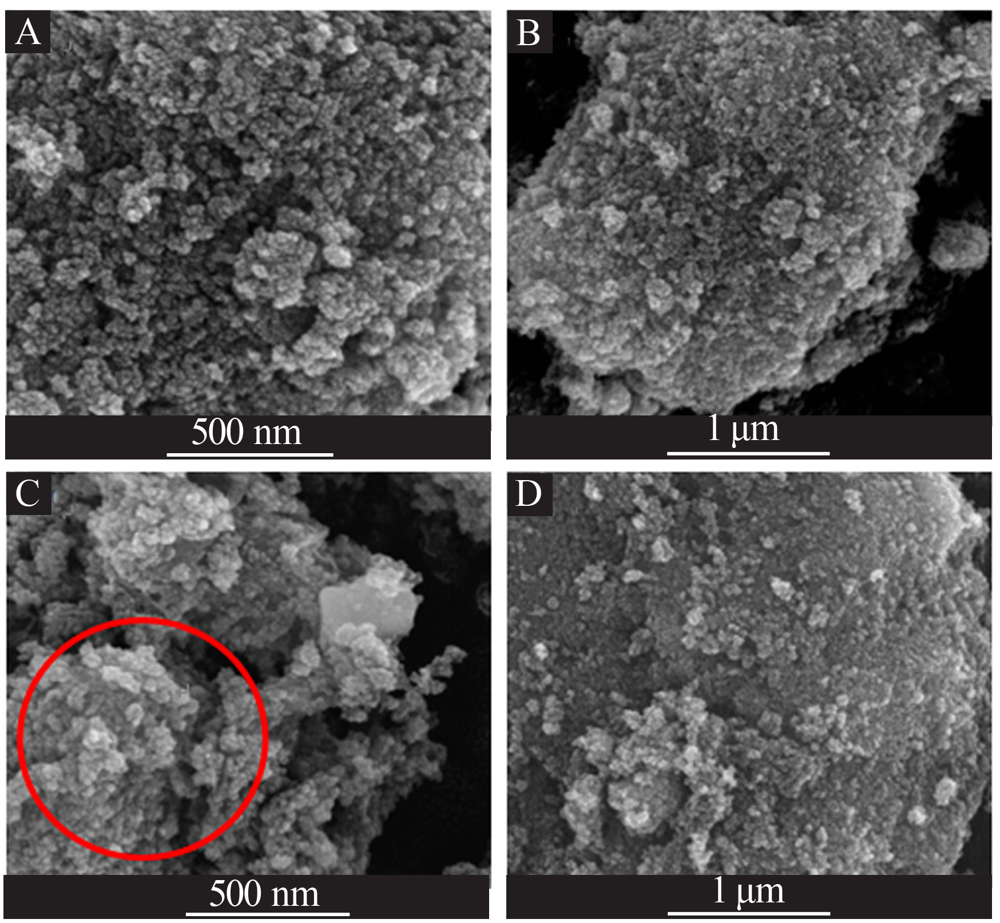
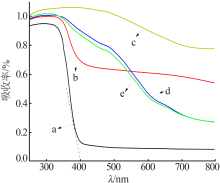
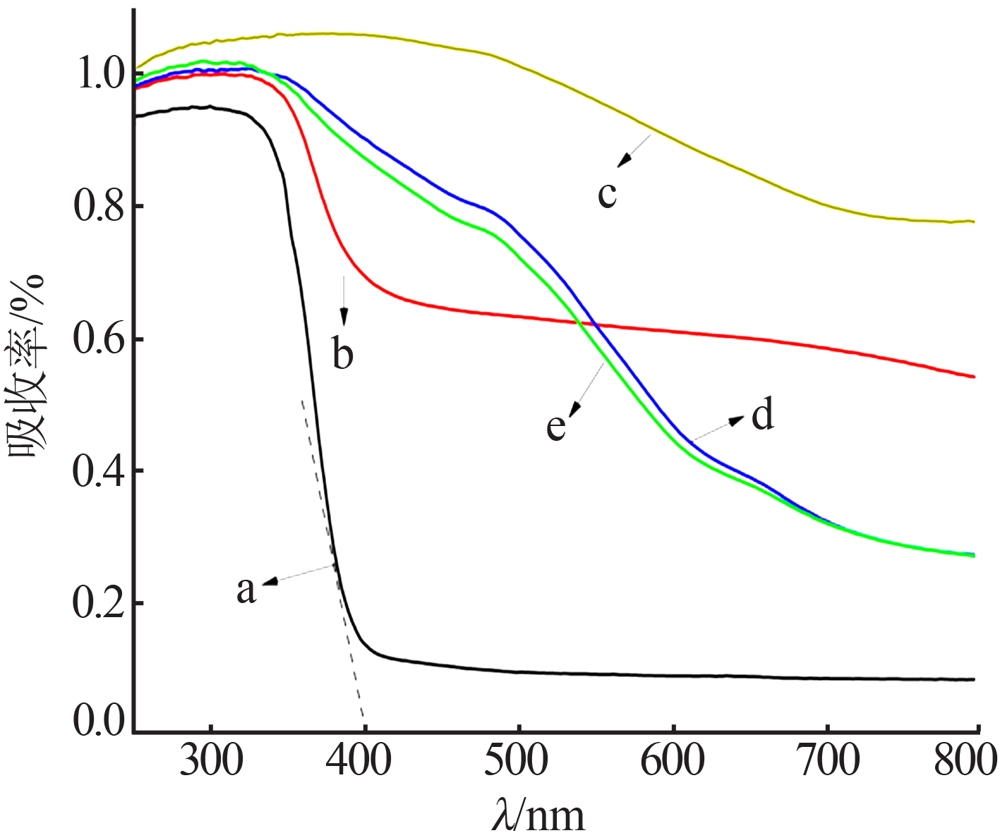
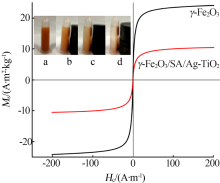
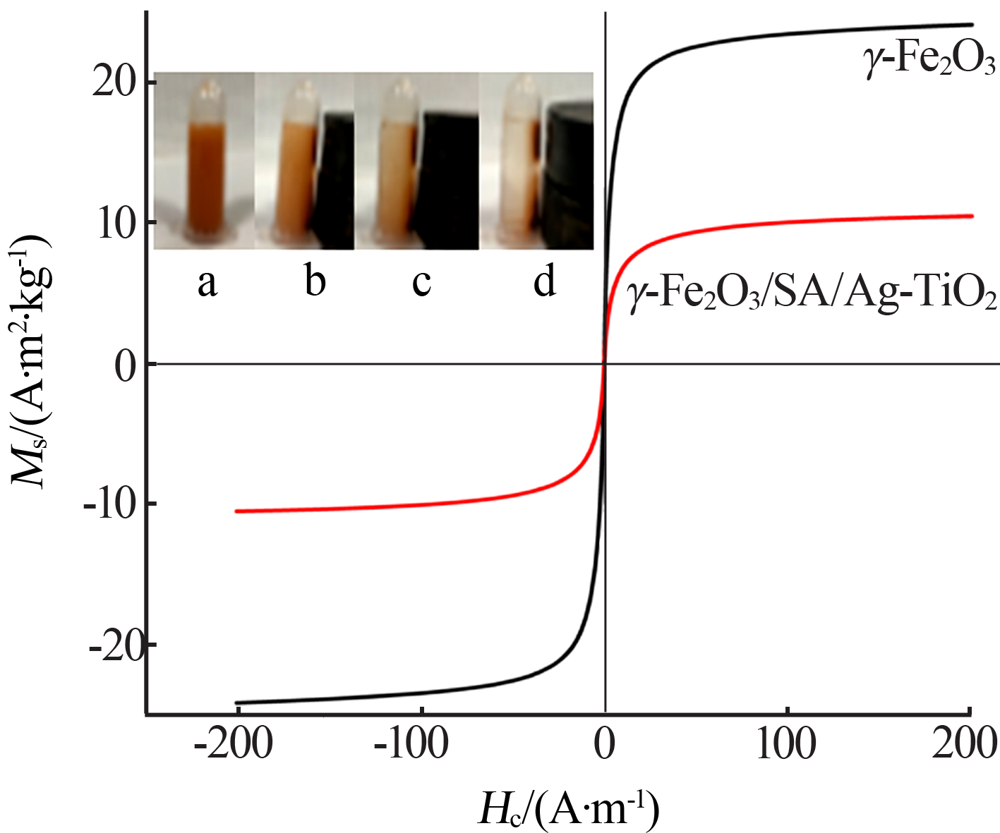
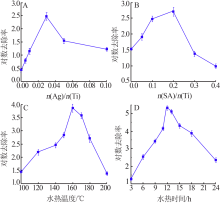
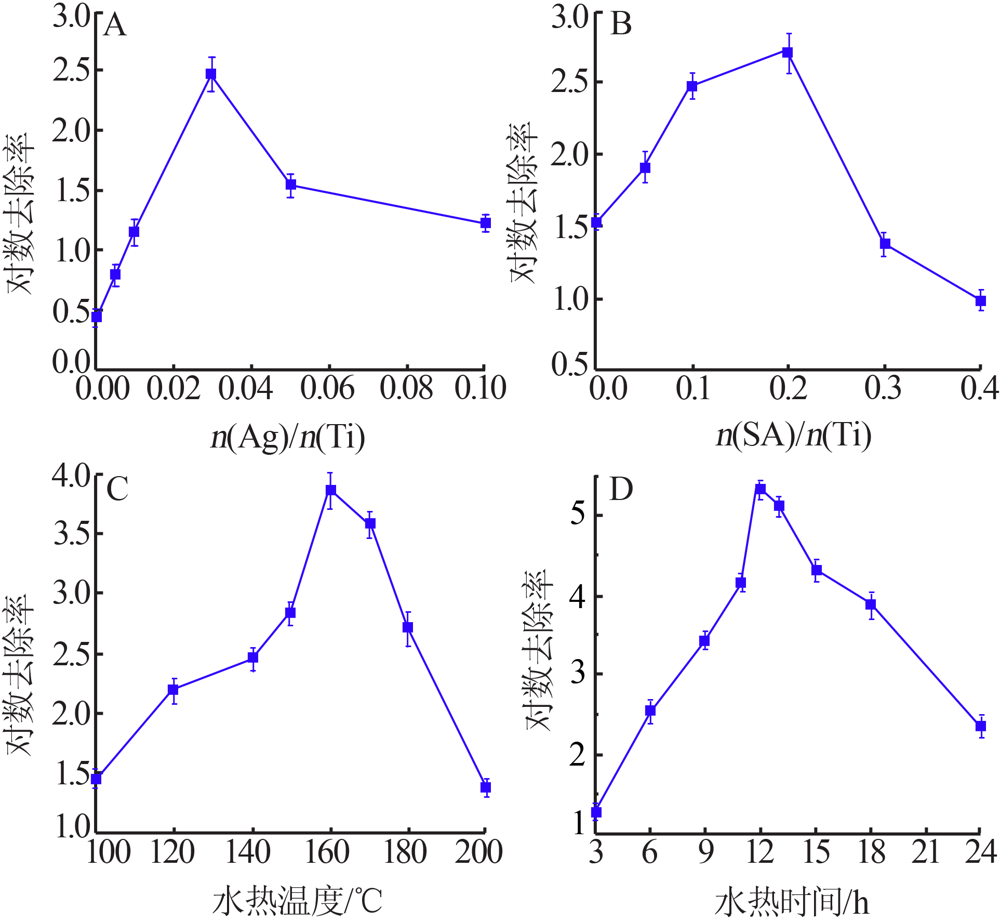
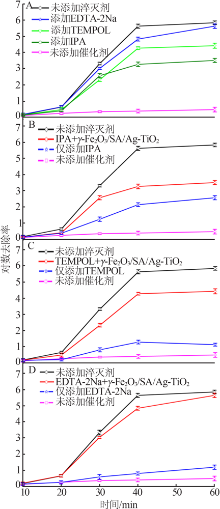
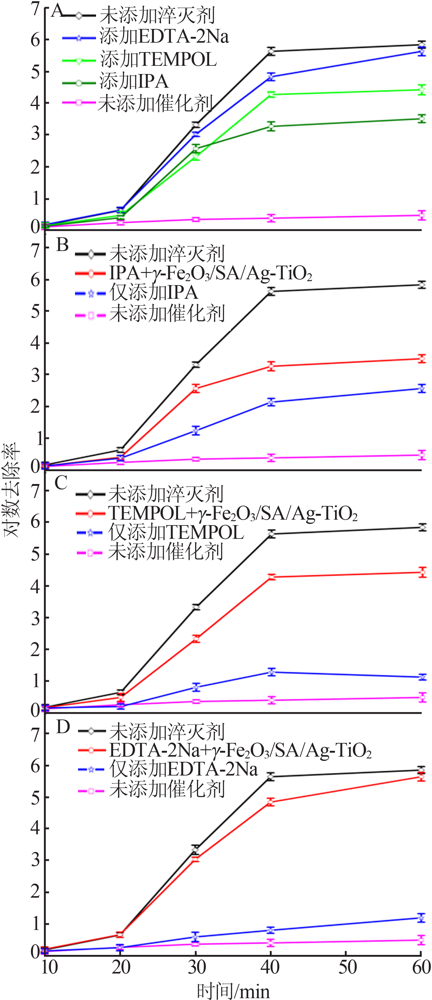
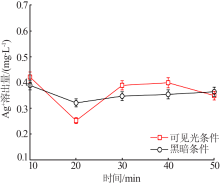
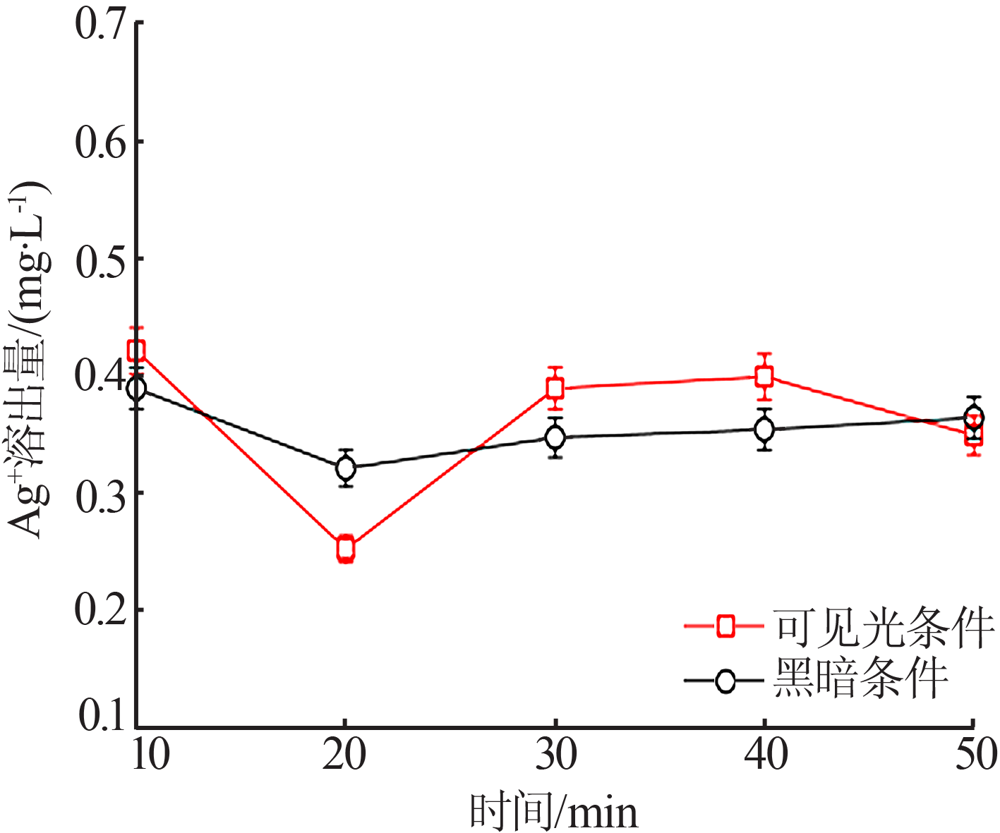
| [1] |
SU H, LIU Y, PAN C, et al. Persistence of antibiotic resistance genes and bacterial community changes in drinking water treatment sys- sem:From drinking water source to tap water[J]. Science of The To- tal Environment, 2018, 616-617:453-461.
|
| [2] |
MATSUNAGA T, TOMODA R, NAKAJIMA T, et al. Photoelectroche- mical sterilization of microbial-cells by semiconductor powders[J]. FEMS Microbiology Letters, 1985, 29(1/2):211-214.
doi: 10.1111/fml.1985.29.issue-1-2
|
| [3] |
OUYANG X, LI X, YAN H, et al. Preparation and characterization of nanosized TiO2 powders by gaseous detonation method[J]. Mate- rials Science and Engineering B:Advanced Functional Solid-State Materials, 2008, 153(1/2/3):21-24.
|
| [4] |
DESHMUKH S P, PATIL S M, MULLANI S B, et al. Silver nanopar- ticles as an effective disinfectant:A review[J]. Materials Science & Engineering C:Materials for Biological Applications, 2019, 97:954-965.
|
| [5] |
李萌, 贺晓静, 王会珍, 等. Ag-TiO2纳米棒阵列的抗菌性和光催化性能研究[J]. 表面技术, 2016, 45(5):181-186.
|
| [6] |
王国军. 山梨酸(钾)的性能及其应用[J]. 中国食品, 2011(11):56-58.
|
| [7] |
MAHMOODI V, BASTAMI T R, AHMADPOUR A. Solar energy ha- rvesting by magnetic-semiconductor nanoheterostructure in water treatment technology[J]. Environmental Science and Pollution Re- search, 2018, 25(9):8268-8285.
|
| [8] |
SANZONE G, ZIMBONE M, CACCIATO G, et al. Ag/TiO2 nanoco- mposite for visible light-driven photocatalysis[J]. Superlattices and Microstructures, 2018, 123:394-402.
doi: 10.1016/j.spmi.2018.09.028
|
| [9] |
YANG Y, LIU E, DAI H, et al. Photocatalytic activity of Ag-TiO2- graphene ternary nanocomposites and application in hydrogen evolu- tion by water splitting[J]. International Journal of Hydrogen Ener- gy, 2014, 39(15):7664-7671.
|
| [10] |
YE L, LIU J, GONG C, et al. Two different roles of metallic Ag on Ag/AgX/BiOX(X=Cl,Br) visible light photocatalysts:Surface pl- asmon resonance and Z-scheme bridge[J]. ACS Catalysis, 2012, 2(8):1677-1683.
doi: 10.1021/cs300213m
|
| [11] |
KOLI V B, DHODAMANI A G, DELEKAR S D, et al. In situ sol- gel synjournal of anatase TiO2-MWCNTs nanocomposites and their photocatalytic applications[J]. Journal of Photochemistry and Pho- tobiology A:Chemistry, 2017, 333:40-48.
|
| [12] |
BAGHERI S, HIR Z A M, YOUSEFI A T, et al. Progress on mesopo- rous titanium dioxide:Synjournal,modification and applications[J]. Microporous and Mesoporous Materials, 2015, 218:206-222.
doi: 10.1016/j.micromeso.2015.05.028
|
| [13] |
WILKE C M, WUNDERLICH B, GAILLARD J, et al. Synergistic bacterial stress results from exposure to nano-Ag and nano-TiO2 mixtures under light in environmental media[J]. Environmental Science & Technology, 2018, 52(5):3185-3194.
doi: 10.1021/acs.est.7b05629
|
| [14] |
李静, 倪刚, 韩影. 磁性γ-Fe2O3/La/Bi2WO6复合材料的制备及其光催化性能的研究[J]. 化学研究与应用, 2017, 29(2):227-231.
|
| [15] |
WEI W, YU D, HUANG Q. Preparation of Ag/TiO2 nanocomposites with controlled crystallization and properties as a multifunctional material for SERS and photocatalytic applications[J]. Spectrochi- mica Acta Part A:Molecular and Biomolecular Spectroscopy, 2020, 243.Doi: 10.1016/j.saa.2020.118793.
doi: 10.1016/j.saa.2020.118793
|
| [16] |
黄奇峰, 高淑梅, 陈国庆, 等. 山梨酸钾的三维荧光光谱特性分析[J]. 江南大学学报:自然科学版, 2012, 11(3):336-340.
|
| [17] |
SUWANNARUANG T, RIVERA K K P, NERAMITTAGAPONG A, et al. Effects of hydrothermal temperature and time on uncalcin- ed TiO2 synjournal for reactive red 120 photocatalytic degrada- tion[J]. Surface & Coatings Technology, 2015, 271:192-200.
doi: 10.1016/j.surfcoat.2014.12.041
|
| [18] |
艾翠玲, 李凌云, 王文骄, 等. β-In2S3的水热合成及合成条件对其光催化降解土霉素的影响[J]. 环境化学, 2017, 36(5):1112-1121.
|
| [19] |
徐正侠, 杨继涛, 刘康, 等. 溶剂热法合成锐钛矿型二氧化钛纳米晶的形状演化规律[J]. 物理化学学报, 2016, 32(2):581-588.
|
| [20] |
艾翠玲, 吴丽娜, 张嵘嵘, 等. γ-Fe2O3/Ag/TiO2的制备与抗菌性能[J]. 高等学校化学学报, 2019, 40(4):645-651.
|
| [21] |
LIU W, GE H, DING X, et al. Cubic nano-silver-decorated mangan- ese dioxide micromotors:Enhanced propulsion and antibacterial performance[J]. Nanoscale, 2020, 12(38):19655-19664.
doi: 10.1039/D0NR06281B
|
| [22] |
MAI L, WANG D, ZHANG S, et al. Synjournal and bactericidal abi- lity of Ag/TiO2 composite films deposited on titanium plate[J]. App- lied Surface Science, 2010, 257(3):974-978.
|
| [23] |
AZIMI S, NEZAMZADEH-EJHIEH A. Enhanced activity of clino- ptilolite-supported hybridized PbS-CdS semiconductors for the photocatalytic degradation of a mixture of tetracycline and ceph- alexin aqueous solution[J]. Journal of Molecular Catalysis A:Che- mical, 2015, 408:152-160.
|
 ),ZHANG Xiangjun,LIN Qingxian
),ZHANG Xiangjun,LIN Qingxian