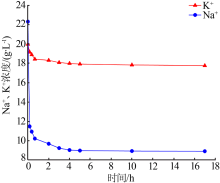
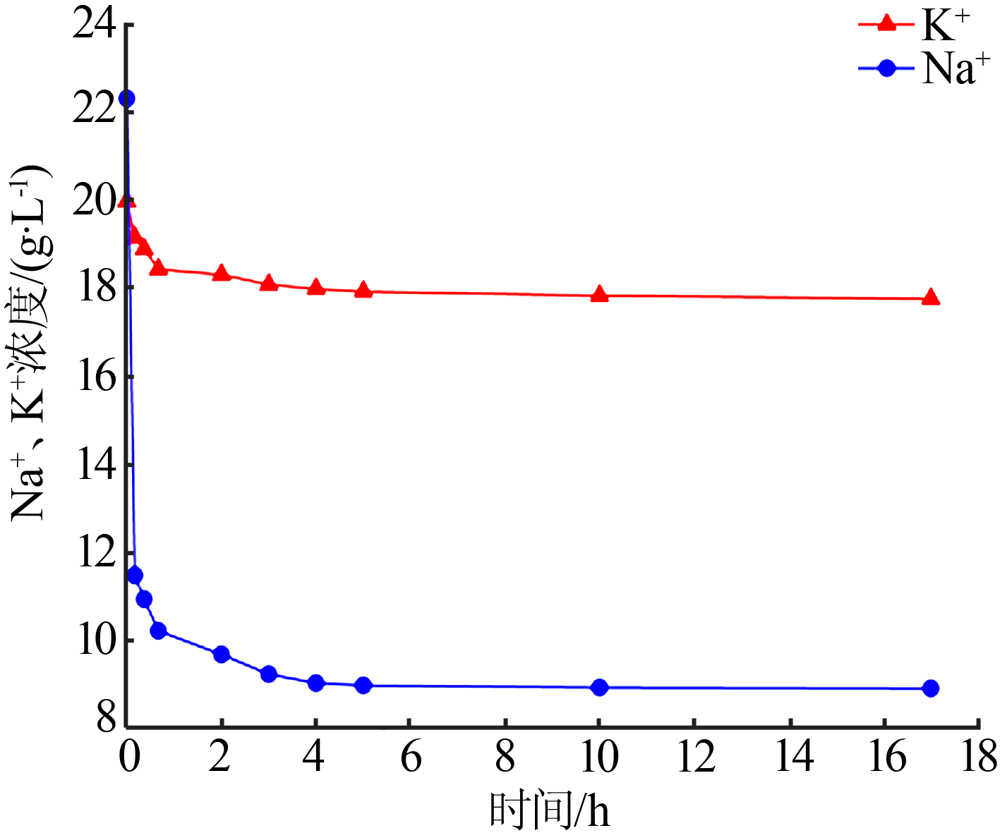
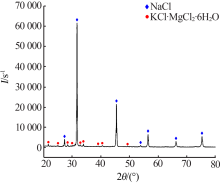
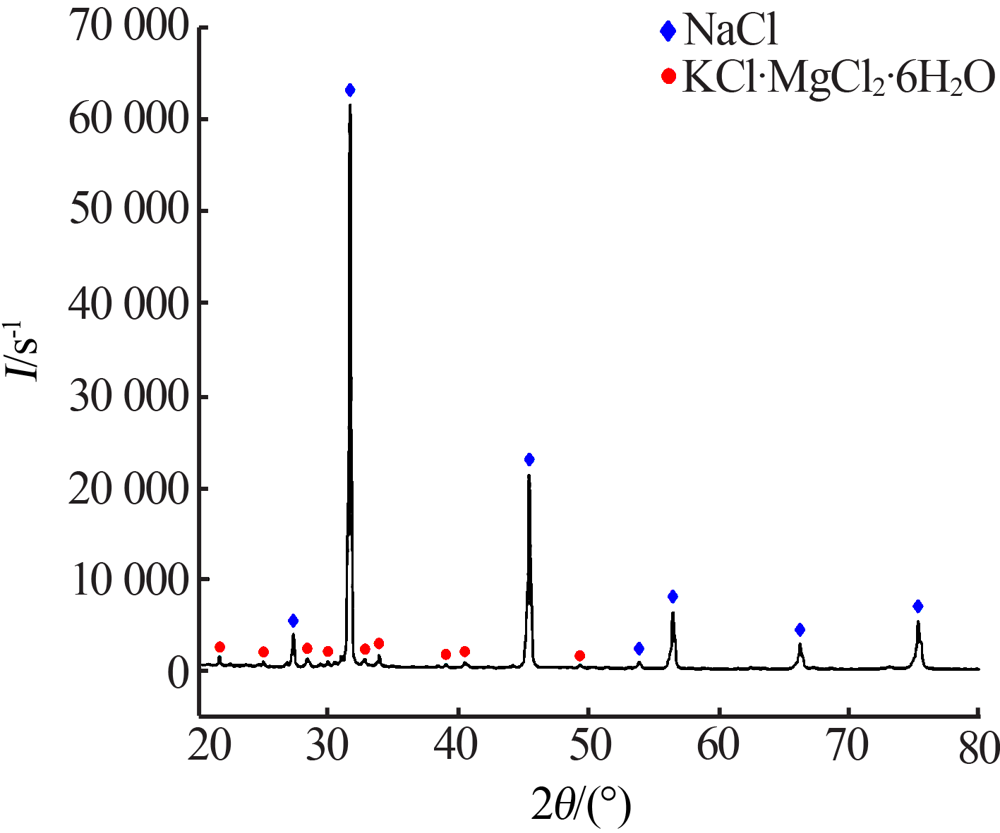
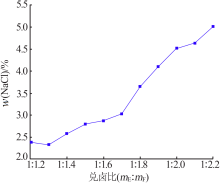
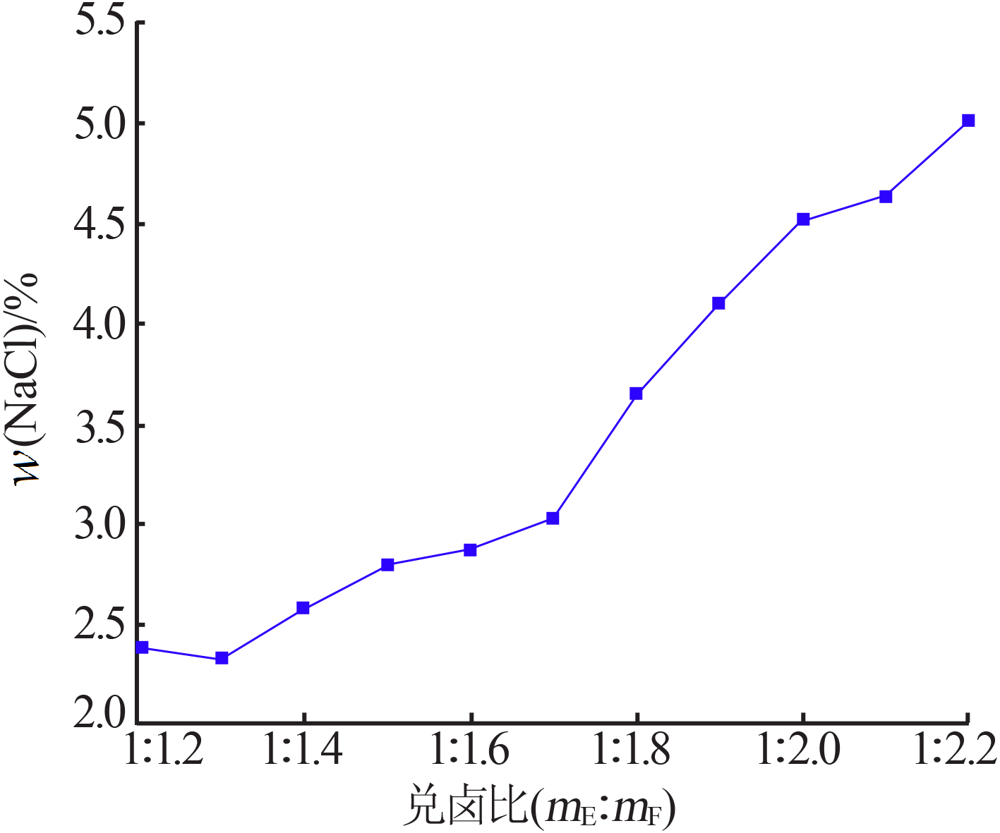
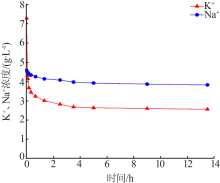
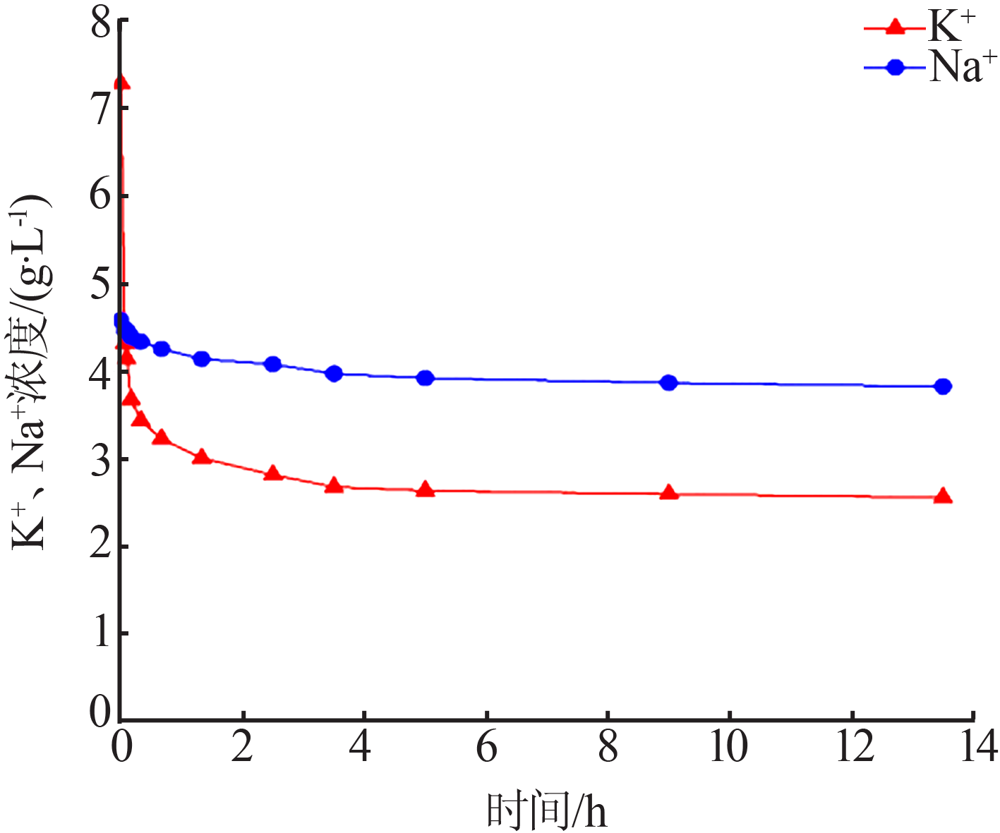
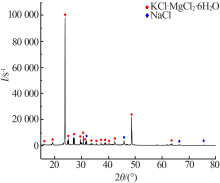
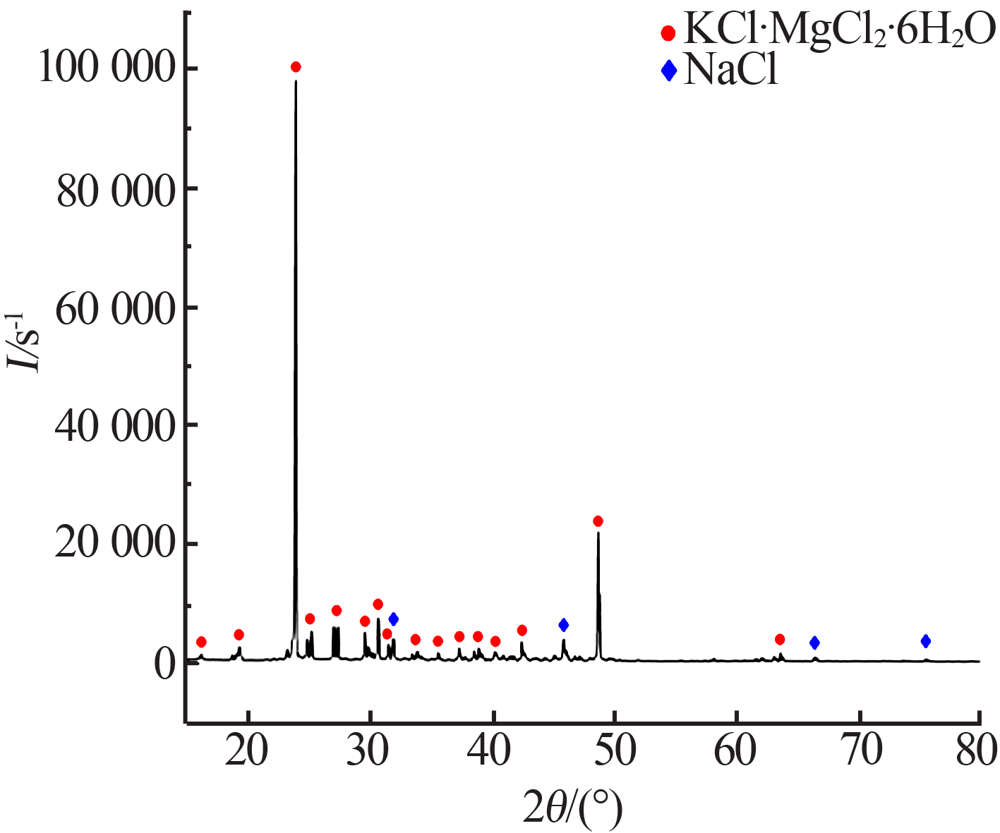
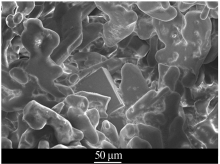
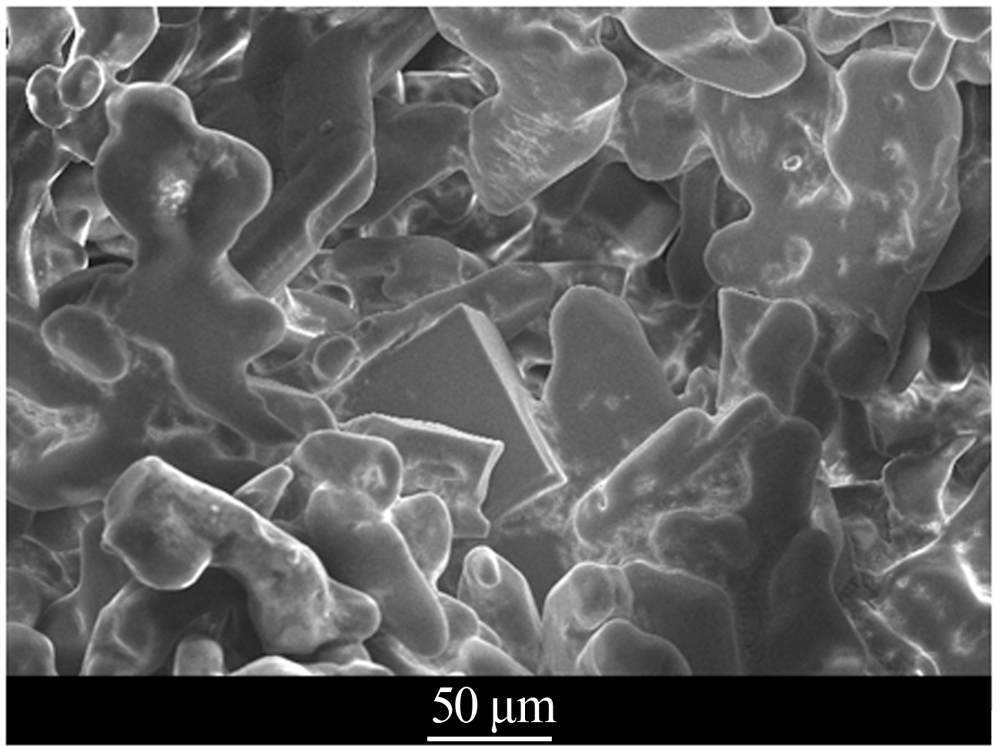
| [1] |
FU Yu, DENG Mi, HUANG Donggen, WAN Jinbao.
Research progress of lithium extraction technology from salt lake brine
[J]. Inorganic Chemicals Industry, 2023, 55(9): 9-16.
|
| [2] |
LI Yan, MA Zhen, SONG Xingfu.
New development advance and industrial development proposals of chloride-type salt lake potassium resources in Qinghai
[J]. Inorganic Chemicals Industry, 2023, 55(8): 84-90.
|
| [3] |
LU Nana, QIN Yaru, MA Shuqing, WANG Qihui, LIU Bing, SHI Chenglong.
Study on extraction of lithium from simulated old brine of salt lake by pyridine ionic liquid system
[J]. Inorganic Chemicals Industry, 2023, 55(7): 45-50.
|
| [4] |
YANG Hongjun, WANG Min, GE Haiwen, QIAO Youmin, QIAO Ziyang.
Study on recycling process of potassium from calcium aluminate fly ash
[J]. Inorganic Chemicals Industry, 2023, 55(10): 121-127.
|
| [5] |
JIN Yinghao, WANG Siyuan, YU Xuefeng, WANG Jinjing, WEI Zhengying, LIU Liwu, TIAN Haifeng, ZHA Fei.
Recombination and application on mixed amine collector(cationic/anionic) for KCl flotation
[J]. Inorganic Chemicals Industry, 2023, 55(10): 93-99.
|
| [6] |
YU Jianguo,SUN Qing,QIU Shengbo,ZHANG Yiren,CHEN Jun.
Lithium resources development supporting national new energy strategy development
[J]. Inorganic Chemicals Industry, 2023, 55(1): 1-14.
|
| [7] |
LIN Yuqing,ZHANG Yiren,QIU Yulong,ZHANG Jiayu,YU Jianguo.
Progress and prospect of membrane technology in lithium extraction from salt lake brine
[J]. Inorganic Chemicals Industry, 2023, 55(1): 33-45.
|
| [8] |
DU Bingxuan,LI Haichao,LIN Zezhong.
Study on preparation of food?grade potassium chloride by one?step adsorption and purification
[J]. Inorganic Chemicals Industry, 2022, 54(9): 96-101.
|
| [9] |
QIE Zhijia,DONG Weibing,DU Jingling,MA Shengkui,WANG Gang.
Study on yield of KCl prepared by cold crystallization of low?sodium carnallite
[J]. Inorganic Chemicals Industry, 2022, 54(5): 72-78.
|
| [10] |
DU Jingling,DONG Weibing,QIE Zhijia,MA Shengkui,TIAN Hongbin,WANG Gang.
Study on effect of trisodium citrate on particle size of potassium chloride crystal prepared by decomposition of low sodium carnallite
[J]. Inorganic Chemicals Industry, 2022, 54(4): 112-118.
|
| [11] |
NIE Zhen,WU Qian,DING Tao,BU Lingzhong,WANG Yunsheng,YU Jiangjiang,HOU Xianhua.
Research progress on industrialization technology of lithium extraction from salt lake brine in China
[J]. Inorganic Chemicals Industry, 2022, 54(10): 1-12.
|
| [12] |
Wan Junfeng,Dong Weibing,Chen Xulong,Du Jingling,Wang Gang.
Coalescence crystallization of large particle KCl and its particle size control
[J]. Inorganic Chemicals Industry, 2021, 53(8): 50-54.
|
| [13] |
HAN Jiahuan,NIE Zhen,FANG Chaohe,WU Qian,CAO Qian,WANG Yunsheng,BO Lingzhong,YU Jiangjiang.
Analysis of existing circumstance of supply and demand on China′s lithium resources
[J]. Inorganic Chemicals Industry, 2021, 53(12): 61-66.
|
| [14] |
Chen Wang,Jiang Lei,Pan Qiaozhen,Yang Weiwei,Zhu Xianrong,Chen Linlin,Zhu Wenshuai.
Research on preparation and adsorption performance of titanium lithium ion sieve
[J]. Inorganic Chemicals Industry, 2021, 53(10): 47-51.
|
| [15] |
Chen Mo,Sha Zuoliang.
Study on new process for producing potassium chloride using cold-decomposition-sieving method
[J]. Inorganic Chemicals Industry, 2021, 53(1): 65-67.
|
 ),Zhang Yu1,2,3,Zhu Junguo1,2,3
),Zhang Yu1,2,3,Zhu Junguo1,2,3