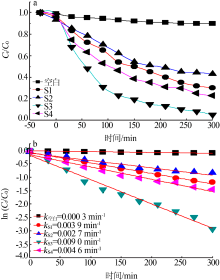
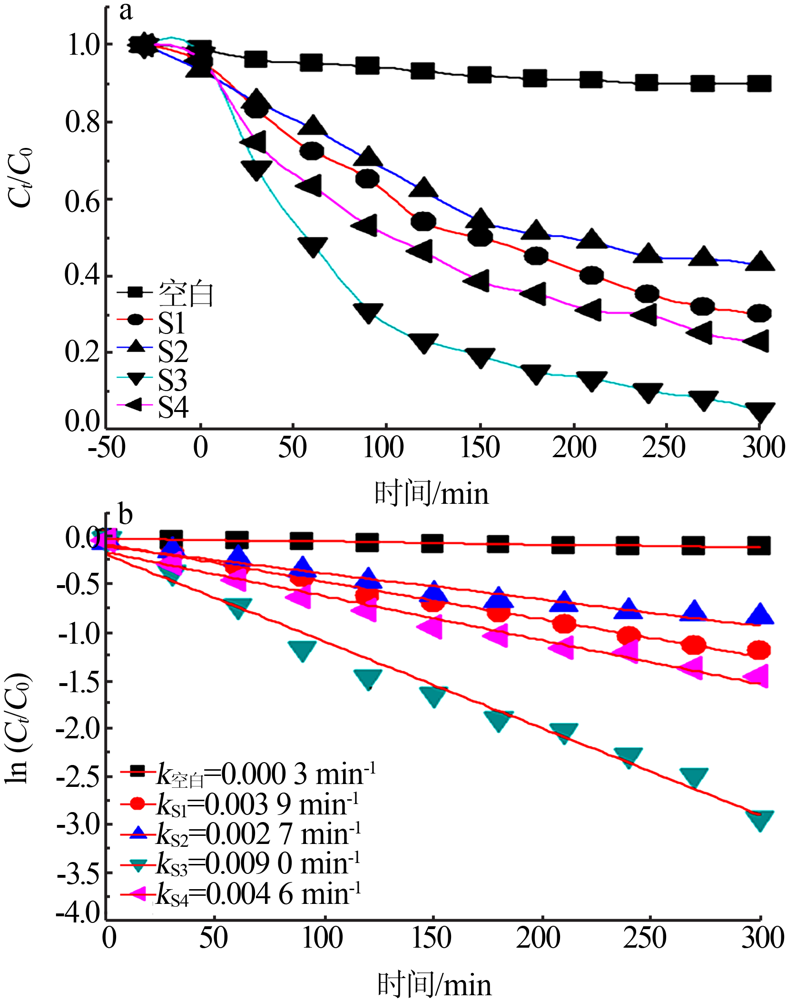
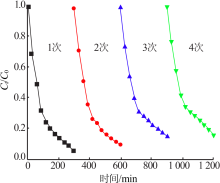
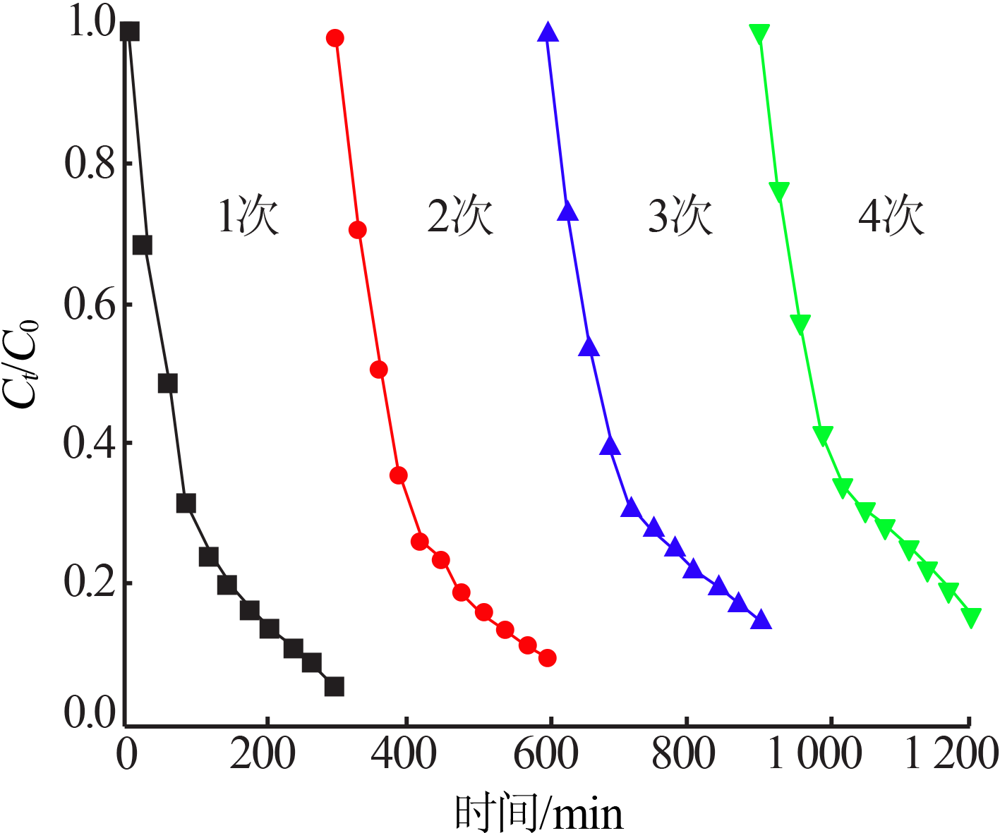
| [1] |
肖姗姗, 毕菲, 赵丽, 等. 氧化亚铜基复合光催化剂的研究进展[J]. 无机盐工业, 2020, 52(1):22-25.
|
| [2] |
Yan Y X, Yang H, Yi Z, et al. Construction of Ag2S@CaTiO3 hetero-junction photocatalysts for enhanced photocatalytic degradation of dyes[J]. Desalination and Water Treatment, 2019, 170:349-360.
doi: 10.5004/dwt
|
| [3] |
安伟佳, 谢婉丽. 碳酸银光催化性能研究[J]. 无机盐工业, 2020, 52(3):107-111.
|
| [4] |
Ochiai T, Fujishima A. Photoelectrochemical properties of TiO2 pho-tocatalyst and its applications for environmental purification[J]. Journal of Photochemistry and Photobiology C:Photochemistry Re-views, 2012, 13(4):247-262.
|
| [5] |
Chen L, Zhang S, Wang L, et al. Photocatalytic activity of Zr:SrTiO3 under UV illumination[J]. Journal of Crystal Growth, 2009, 311(3):735-737.
doi: 10.1016/j.jcrysgro.2008.09.181
|
| [6] |
朱佳新, 熊裕华, 郭锐. 二氧化钛光催化剂改性研究进展[J]. 无机盐工业, 2020, 52(3):24-27.
|
| [7] |
张树立, 徐旭鹏, 陈书锐, 等. 钒掺杂二氧化钛光催化活性研究[J]. 无机盐工业, 2020, 52(8):123-127.
|
| [8] |
Cui W, Chen L, Li J, et al. Ba-vacancy induces semiconductor-like photocatalysis on insulator BaSO4[J]. Applied Catalysis B:Enviro-nmental, 2019, 253:293-299.
|
| [9] |
陈桂光, 骆广生, 杨雪瑞, 等. BaSO4/TiO2复合颗粒制备及其光催化性质[J]. 过程工程学报, 2005, 5(1):69-72.
|
| [10] |
Selvakumar R, Ashok A, Suriyaraj S, et al. Rapid and efficient visi-ble light photocatalytic dye degradation using AFe2O4(A=Ba,Ca and Sr) complex oxides[J]. Materials Science & Engineering B, 2016, 210:43-50.
|
| [11] |
Ay A N, Zümreoglu Karan B, Temel A, et al. Bioinorganic magnetic core-shell nanocomposites carrying antiarthritic agents:Intercala-tion of ibuprofen and glucuronic acid into Mg-Al-layered double hydroxides supported on magnesium ferrite[J]. Inorganic Chemi-stry, 2009, 48:8871-8887.
|
| [12] |
张明, 李新海, 胡启阳, 等. EDTA络合法合成硫酸钡微粒[J]. 中国有色金属学报, 2009, 19(8):1511-1516.
|
| [13] |
Wang S F, Li Q, Zu X T, et al. Phase controlled synjournal of(Mg,Ca,Ba)-ferrite magnetic nanoparticles with high uniformity[J]. Journal of Magnetism and Magnetic Materials, 2016, 419(12):464-475.
doi: 10.1016/j.jmmm.2016.06.056
|
| [14] |
杨晓红. MgAl2O4:Ce荧光粉辐照合成及发光机理研究[J]. 无机盐工业, 2019, 51(9):30-35.
|
| [15] |
Salah N, Habib S S, Khan Z H, et al. Nanoparticles of BaSO4:Eu for heavy-dose measurements[J]. Journal of Luminescence, 2009, 129(3):192-196.
doi: 10.1016/j.jlumin.2008.09.012
|
| [16] |
Gao H, Yang H, Wang S. Comparative study on optical and electro-chemical properties of MFe2O4(M=Mg,Ca,Ba) nanoparticles[J]. Transactions Indian Ceramic Society, 2018, 77(3):1-11.
doi: 10.1080/0371750X.2017.1417059
|
| [17] |
Tachikawa T, Fujitsuka M, Majima T. Mechanistic insight into the TiO2 photocatalytic reactions:Design of new photocatalysts[J]. Journal of Physical Chemistry C, 2007, 111(14):5259-5275.
doi: 10.1021/jp069005u
|
| [18] |
Arai T, Yanagida M, Konishi Y, et al. Efficient complete oxidation of acetaldehyde into CO2 over CuBi2O4/WO3 composite photocatalyst under visible and UV light irradiation[J]. Journal of Physical Chemistry C, 2007, 111(21):7574-7577.
doi: 10.1021/jp0725533
|
 ),Hou Fang1,Gao Xiping2
),Hou Fang1,Gao Xiping2