| [1] |
Lin F, Dennis N, Li Y Y, et al. Metal segregation in hierarchically structured cathode materials for high-energy lithium batteries[J]. Nature Energy, 2016,1(1):1-8.
|
| [2] |
Ding X K, Luo D, Cui J X, et al. An ultra-long-life lithium-rich Li1.2Mn0.6Ni0.2O2 cathode by three-in-one surface modification for lit-hium-ion batteries[J]. Angewandte Chemie, 2020,59(20):7778-7782.
|
| [3] |
何爱珍. 铝掺杂对Li1.2Ni0.2Mn0.6O2结构和电化学性能的影响[J]. 无机盐工业, 2017,49(7):74-77.
|
| [4] |
Nayak P K, Erickson E M, Schipper F, et al. Review on challenges and recent advances in the electrochemical performance of high ca-pacity Li-and Mn-rich cathode materials for Li-ion batteries[J]. Advanced Energy Materials, 2018,8(8).Doi: 10.1002/aenm.201702397.
|
| [5] |
Nayak P K, Grinblat J, Levi M, et al. Al doping for mitigating the capacity fading and voltage decay of layered Li and Mn-rich catho-des for Li-ion batteries[J]. Advanced Energy Materials, 2016,6(8). Doi: 10.1002/aenm.201502398.
|
| [6] |
Wang C C, Manthiram A. Influence of cationic substitutions on the first charge and reversible capacities of lithium-rich layered oxide cathodes[J]. Journal of Materials Chemistry A, 2013,1(35):10209-10217.
doi: 10.1039/c3ta11703k
|
| [7] |
Guo H C, Xia Y G, Zhao H, et al. Stabilization effects of Al doping for enhanced cycling performances of Li-rich layered oxides[J]. Ceramics International, 2017,43(16):13845-13852.
doi: 10.1016/j.ceramint.2017.07.107
|
| [8] |
Chong S K, Wu Y F, Chen Y Z, et al. A strategy of constructing sphe-rical core-shell structure of Li1.2Ni0.2Mn0.6O2@Li1.2Ni0.4Mn0.4O2 catho-de material for high-performance lithium-ion batteries[J]. Journal of Power Sources, 2017,356:153-162.
doi: 10.1016/j.jpowsour.2017.04.081
|
| [9] |
Han Y, Shan X, Zhu G, et al. Hierarchically assembled LiNi0.8Co0.1Mn0.1O2 secondary particles with high exposure of {010} plane synthesized via co-precipitation method[J]. Electrochimica Acta, 2020,329.Doi: 10.1016/j.electacta.2019.135057.
|
| [10] |
Dannehl N, Steinmuüller S O, Szabó D V, et al. High-resolution sur-face analysis on aluminum oxide coated Li1.2Mn0.55Ni0.15Co0.1O2 with improved capacity retention[J]. ACS applied materials & interfac-es, 2018,10(49):43131-43143.
|
| [11] |
Zhang J C, Zhang H, Gao R, et al. New insights into the modifica-tion mechanism of Li-rich Li1.2Mn0.6Ni0.2O2 coated by Li2ZrO3[J]. Physical Chemistry Chemical Physics, 2016,18(19):13322-13331.
doi: 10.1039/C6CP01366J
|
| [12] |
Pei Y, Chen Q, Xiao Y C, et al. Understanding the phase transitions in spinel-layered-rock salt system:Criterion for the rational design of LLO/spinel nanocomposites[J]. Nano Energy, 2017,40:566-575.
doi: 10.1016/j.nanoen.2017.08.054
|
| [13] |
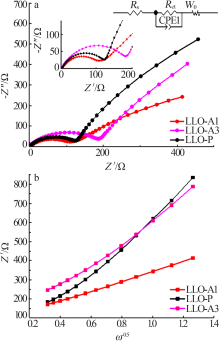
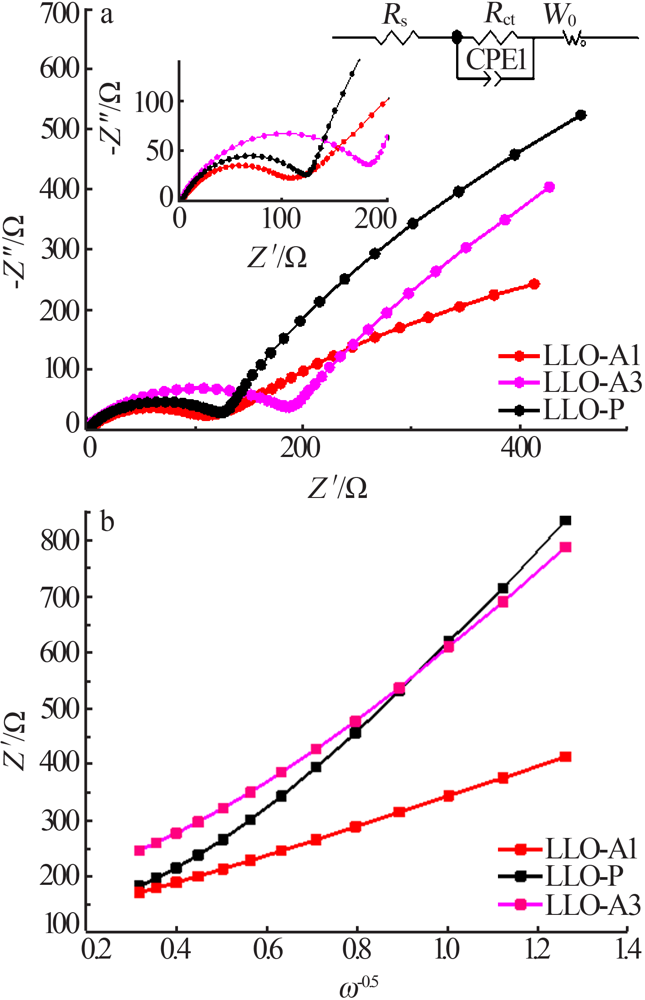
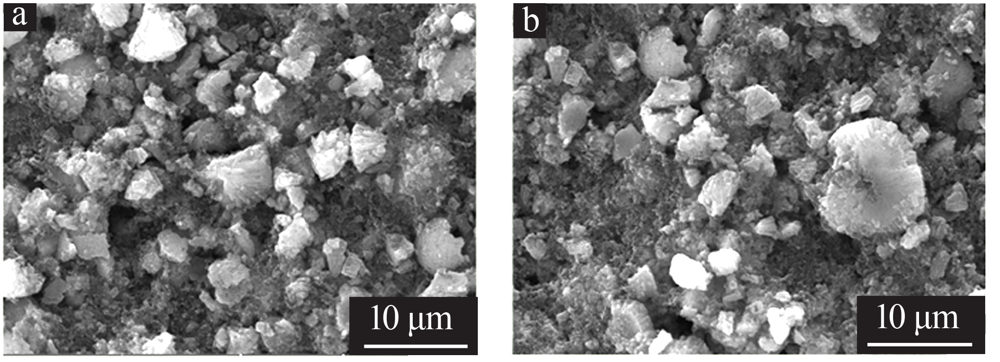
王雅思, 吴锋, 张存中. 富锂锰基材料充电过程中的动态 Rct[J]. 电源技术, 2016(3):503-506.
|
| [14] |
Lai X, Hu G, Peng Z, et al. Surface structure decoration of high ca-pacity Li1.2Mn0.54Ni0.13Co0.13O2 cathode by mixed conductive coating of Li1.4Al0.4Ti1.6(PO4)3 and polyaniline for lithium-ion batteries[J]. Journal of Power Sources, 2019,431:144-152.
doi: 10.1016/j.jpowsour.2019.05.044
|
 ),Chen Yanxiao1,Guo Xiaodong1(
),Chen Yanxiao1,Guo Xiaodong1( ),Xiang Wei2
),Xiang Wei2