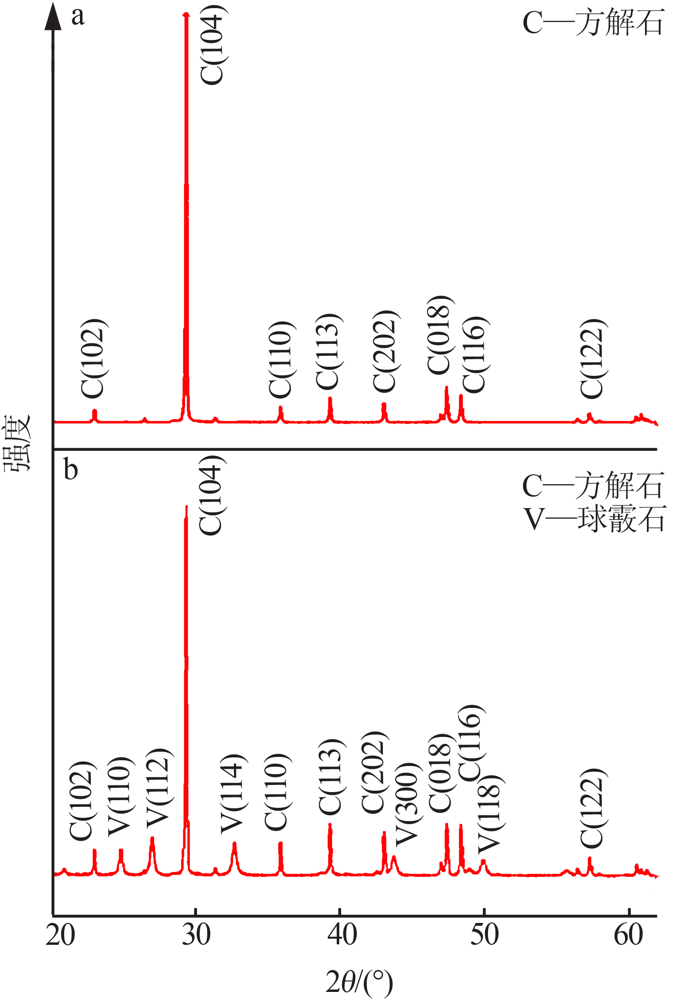
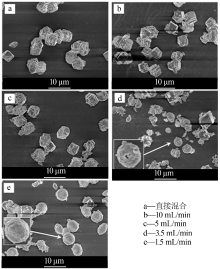
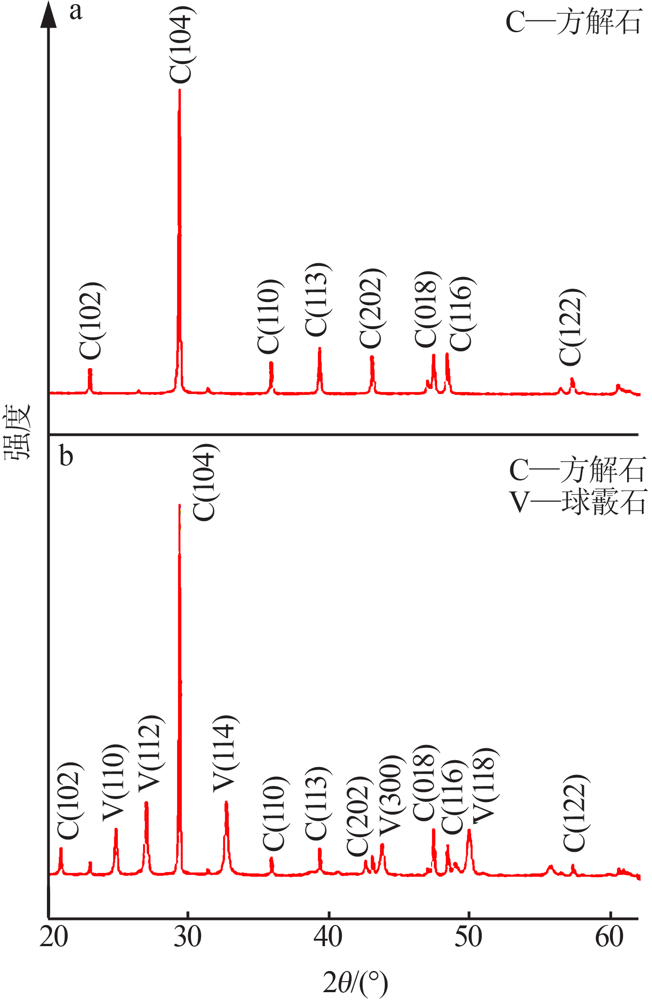
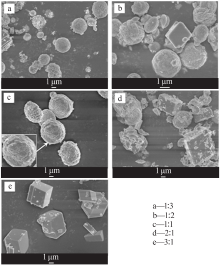
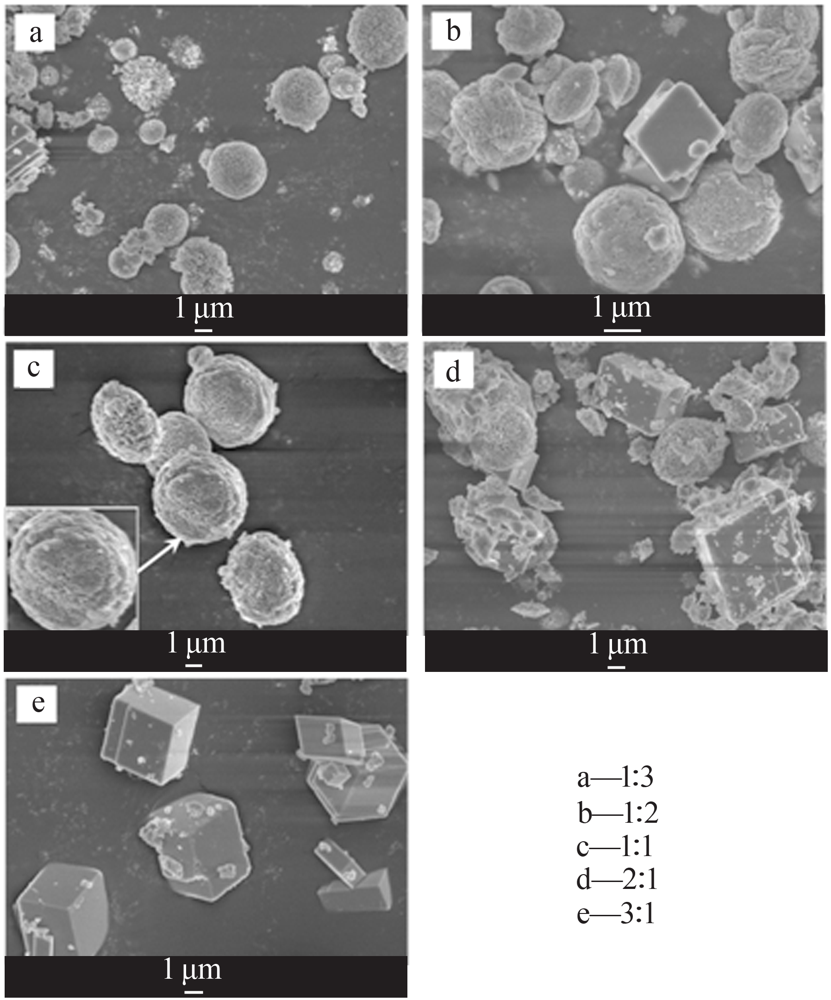
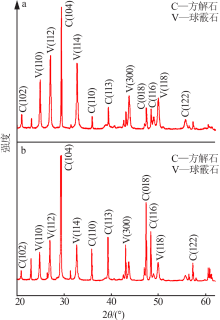
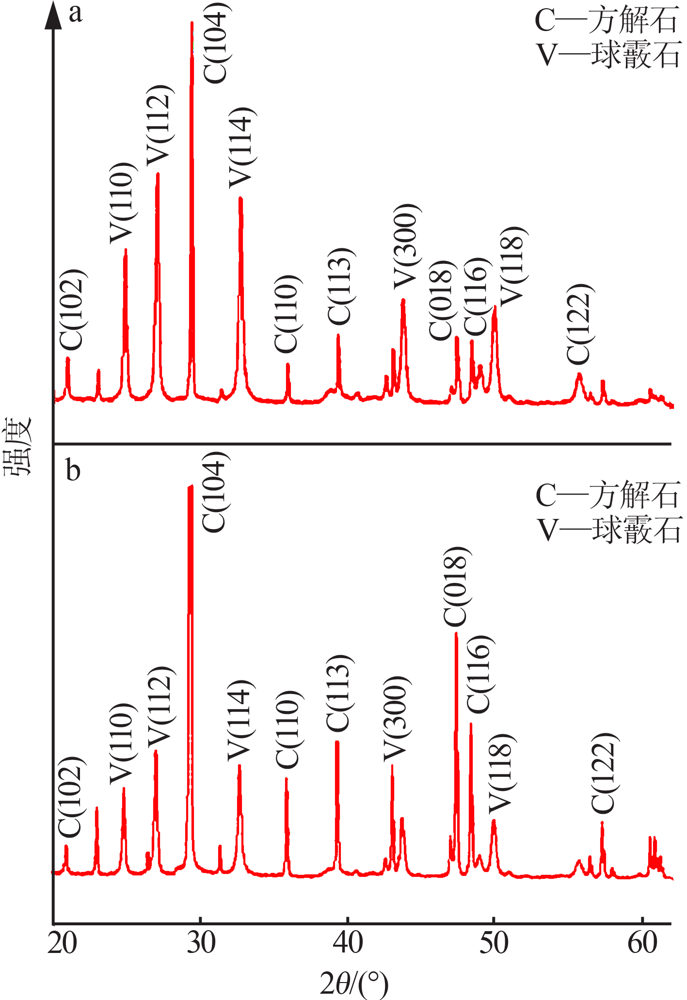
| [1] |
李梦维, 赵昌明, 程铁欣, 等. 十二烷基苯磺酸钠对碳酸钙结晶行为的影响[J]. 高等学校化学学报, 2018,39(11):2380-2385.
|
| [2] |
周绿山, 赖川, 王芬, 等. 多孔碳酸钙的制备及应用研究进展[J]. 化工进展, 2018,37(1):166-174.
|
| [3] |
陈银霞, 赵改青, 王晓波. 聚合物控制碳酸钙晶型、形貌的研究[J]. 化学进展, 2009,21(7):1619-1625.
|
| [4] |
吴刚, 王小锋, 郎雷鸣, 等. 溶剂和溶液酸碱性对碳酸钙晶型和形状影响研究[J]. 化学研究与应用, 2010,22(10):1310-1314.
|
| [5] |
黄文艺, 马蓝宇, 程昊, 等. 氯化铝对碳酸钙结晶形貌的影响[J]. 无机盐工业, 2018,50(1):20-23.
|
| [6] |
王君, 蔡杰慧, 韦雄雄, 等. CaCO3微球的制备及表征[J]. 高校化学工程学报, 2019,33(1):199-206.
|
| [7] |
郑天文, 陈雪梅. 碳酸钙空心微球的合成及其生成机理[J]. 化工进展, 2017,36(3):989-995.
|
| [8] |
王宇轩, 徐颖, 王东平, 等. 球霰石的性质及其应用进展[J]. 安徽理工大学学报:自然科学版, 2017,37(2):76-80.
|
| [9] |
王俊, 王艳玲, 施伟光, 等. 可溶性添加剂对碳酸钙晶型及形貌控制的研究进展[J]. 硅酸盐通报, 2015,34(12):117-122.
|
| [10] |
谭婷婷, 仲剑初. 球形碳酸钙的控制合成研究[J]. 无机盐工业, 2020,51(12):30-34.
|
| [11] |
张晓蕾, 邱勇波. 球霰石碳酸钙的制备及其稳定性研究[J]. 无机盐工业, 2018,50(2):50-53.
|
| [12] |
郑天文, 陈雪梅. 球霰石碳酸钙微球的合成及其机理[J]. 材料科学与工程学报, 2018,36(3):358-364.
|
| [13] |
丁红霞, 尹晓爽, 杨文忠. 聚合物与表面活性剂混合模板调控碳酸钙结晶[J]. 人工晶体学报, 2011,40(1):258-265.
|
| [14] |
张娟. 碳酸钙的仿生合成及形貌表征[D]. 烟台:烟台大学, 2008.
|
| [15] |
王农, 王兴权, 杨利娟, 等. 添加剂对纳米碳酸钙形貌与粒度的影响[J]. 无机盐工业, 2013,45(2):23-26.
|
| [16] |
张慧, 尹晓爽, 杨文忠. 链状与环状多糖调控CaCO3的仿生合成[J]. 南京工业大学学报:自然科学版, 2010,32(1):50-54.
|
| [17] |
马洁, 李春忠, 陈雪花, 等. 糖类添加剂对纳米碳酸钙形貌的影响[J]. 华东理工大学学报:自然科学版, 2005,31(6):817-820.
|
| [18] |
刘飞, 袁铭鸿, 曹建新. 利用电石渣制备碳酸钙晶须的初步研究[J]. 贵州大学学报:自然科学版, 2010,27(2):126-128.
|
| [19] |
许肖云, 梁兵, 陈丽萍, 等. 无添加剂条件下文石型碳酸钙晶须制备及影响因素研究[J]. 化学研究与应用, 2012,24(6):953-958.
|
 ),Hu Chenxin2,Peng Jianlong2,Zhong Xing2(
),Hu Chenxin2,Peng Jianlong2,Zhong Xing2( )
)