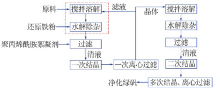
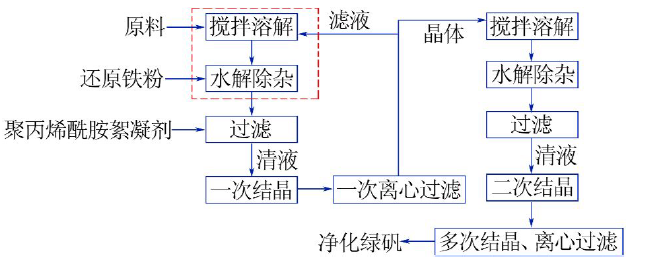
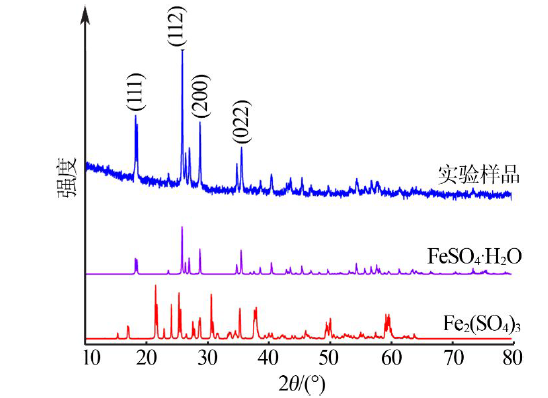
| [1] |
崔佳娜, 任慧莉. 我国钛白粉生产工艺技术的发展及比较[J]. 稀有金属与硬质合金, 2013,41(4):14-17.
|
| [2] |
李化全. 硫酸法钛白粉20年发展浅析[J]. 中国涂料, 2019,34(9):7-10.
|
| [3] |
毕胜. 2018年中国钛白粉行业状况及发展[J]. 钢铁钒钛, 2018,39(6):1-4.
|
| [4] |
郝朝辉, 邵瑞林, 刘晓鸽, 等. 钛白粉生产工艺技术进展[J]. 工程技术:全文版, 2016,5(46):70-71.
|
| [5] |
Duckhyun Kim, Jae Kwan Lee, Sang Ook Kang, et al. Molecular eng-ineering of organic dyes containing N-aryl carbazole moiety for so-lar[J]. Tetrahedron, 2007,63(9):1913-1922.
|
| [6] |
赵蕾, 王富. 钛白副产物硫酸亚铁的精制研究[J]. 山东化工, 2018,47(1):32-33.
|
| [7] |
于耀杰. 硫酸法钛白粉生产副产品废酸的综合利用[J]. 化工设计通讯, 2019,45(5):145,165.
|
| [8] |
郭举. 钛白渣提纯制备电池级硫酸亚铁工艺技术研究[J]. 无机盐工业, 2019,51(8):48-51.
|
| [9] |
钟红梅, 侯德顺. 钛白副产硫酸亚铁的综合利用[J]. 广东化工, 2012,39(15):49-50.
|
| [10] |
任南琪, 周显娇, 郭婉茜, 等. 染料废水处理技术研究进展[J]. 化工学报, 2013,64(1):91-101.
|
| [11] |
Flanagan J, Griffith W P, Skapski A C, et al. The active principle of Caro′s acid,HSO5-:X-ray crystal structure of KHSO5·H2O[J]. Journal of the Chemical Society,Chemical Communications, 1984,23(23):1574-1575.
|
| [12] |
YangShijian, He Hongping, Wu Daqing. Decolorization of methyl-ene blue by heterogeneous Fenton reaction using Fe3-xTixO4(0≤x≤0.78) at neutral pH values[J]. Applied Catalysis B:Environ-mental, 2009,89(3):527-535.
|
| [13] |
宿程远, 郑鹏, 廖黎明, 等. Fe3O4 NPs 类芬顿预处理对活性污泥法处理阿莫西林废水的影响[J]. 化工学报, 2018,69(12):5237-5245.
|
| [14] |
张克宇, 吴鉴, 周朝金, 等. 结晶法提纯钛白副产硫酸亚铁[J]. 有色金属工程, 2017,7(2):10-15.
|
| [15] |
Zong M, Huang Y, Ding X, et al. One-step hydrothermal synjournal and microwave electromagnetic properties of RGO/NiFe2O4 compo-site[J]. Ceramics International, 2014,40(5):6821-6828.
doi: 10.1016/j.ceramint.2013.11.145
|
| [16] |
李长海, 贾冬梅, 张岩, 等. 芬顿法处理阿特拉津合成废水的优化试验[J]. 环境工程, 2017,35(1):55-58.
|
| [17] |
王昶, 薛智芸, 邱泓雨, 等. 蛭石类芬顿催化氧化降解紫丁香醇的研究[J]. 环境工程, 2017,35(4):1-5.
|
| [18] |
Kwan W P, Voelker B M. Rates of hydroxyl radical generation and organic compound oxidation in mineral-catalyzed fenton-like sys-tems[J]. Environmental Science & Technology, 2018,37(6):1150-1158.
pmid: 12680668
|
 ),Zhang Pan1,2,Wang Wei1,2,Xiang Hengli1,2,Ren Genkuan3,Xu Dehua1,2,Zhang Zhiye1,2,Yang Xiushan1,2(
),Zhang Pan1,2,Wang Wei1,2,Xiang Hengli1,2,Ren Genkuan3,Xu Dehua1,2,Zhang Zhiye1,2,Yang Xiushan1,2( )
)