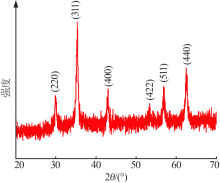
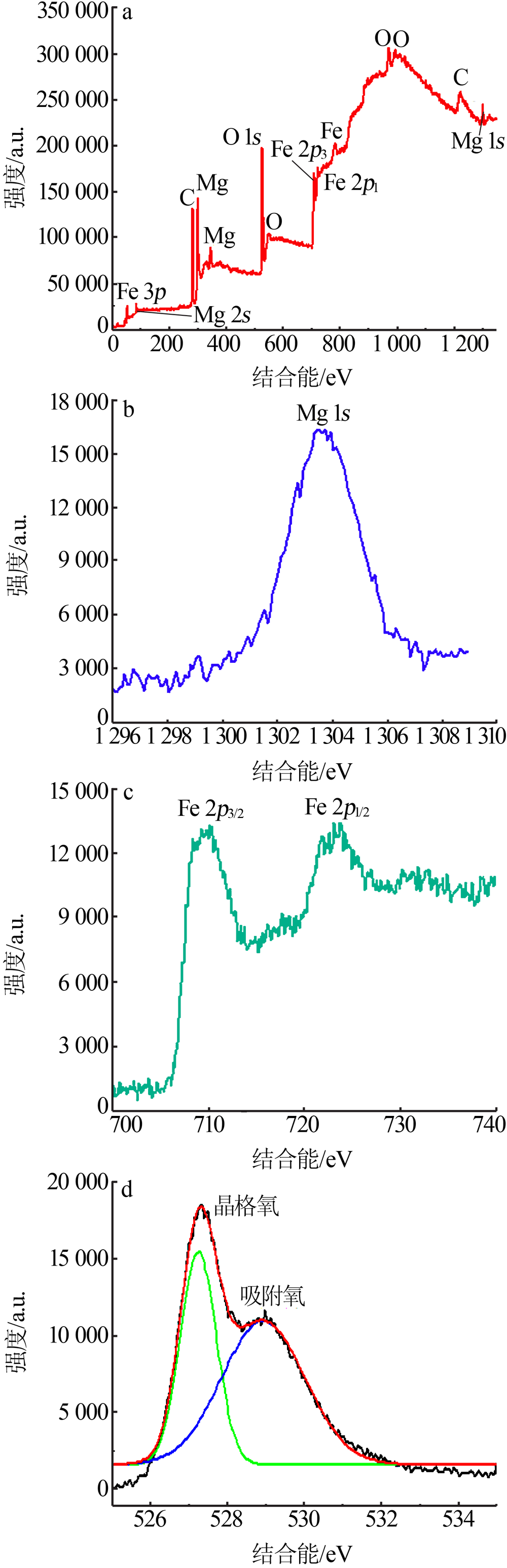
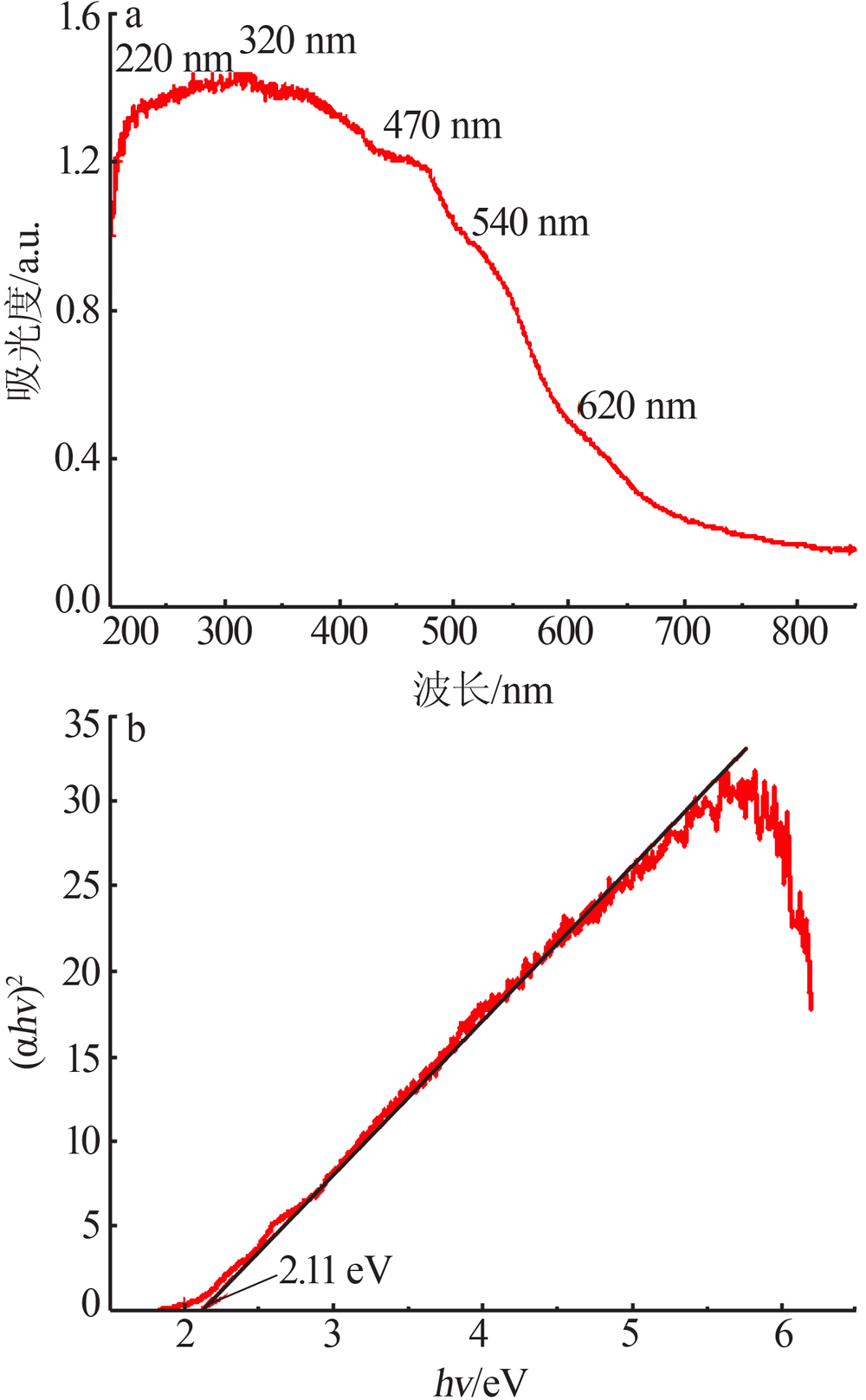
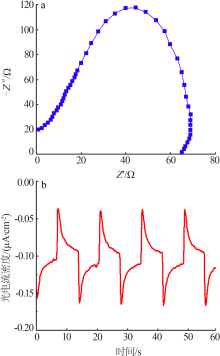
Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (11): 103-107.doi: 10.11962/1006-4990.2019-0653
• Catalytic Materials • Previous Articles
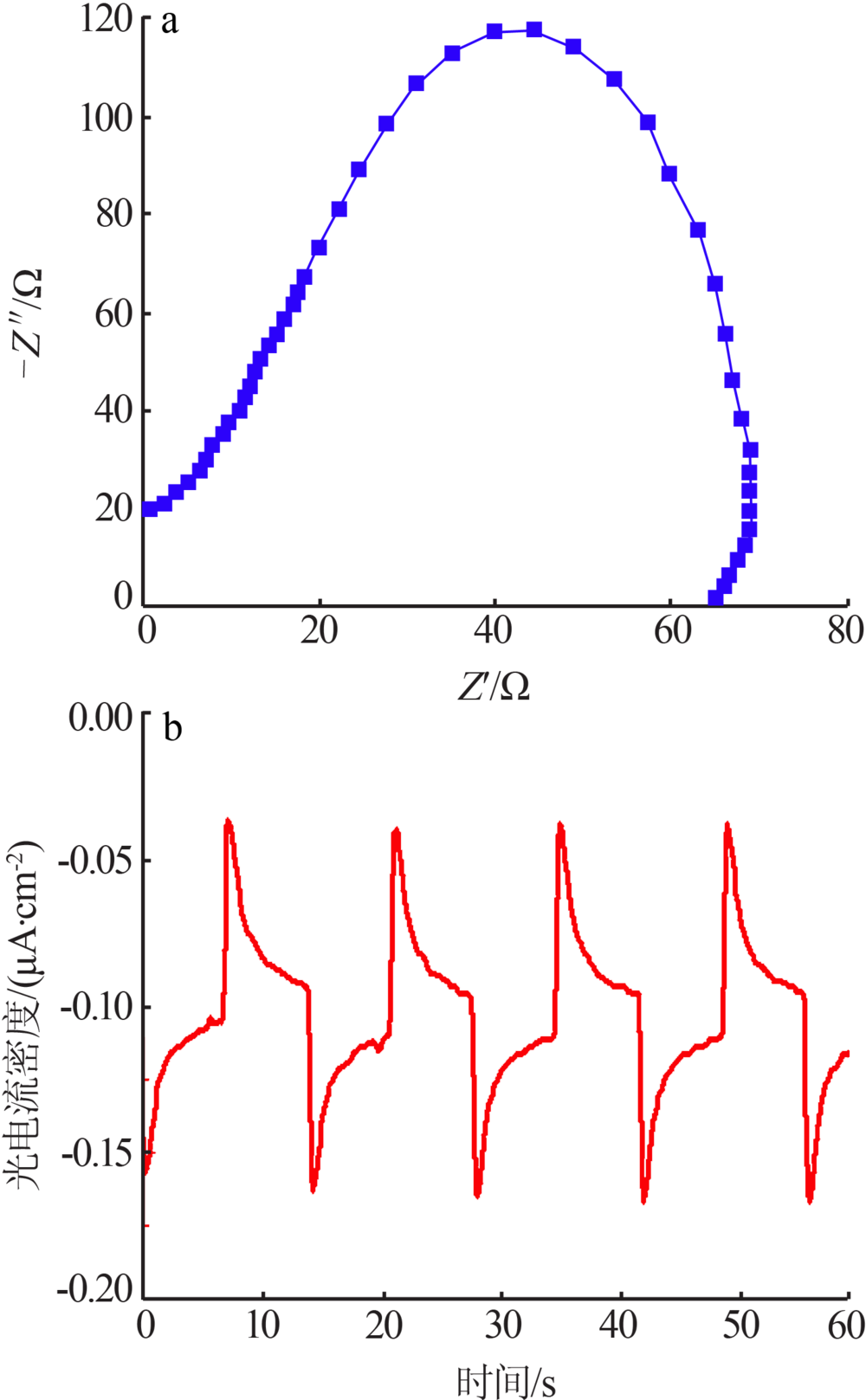
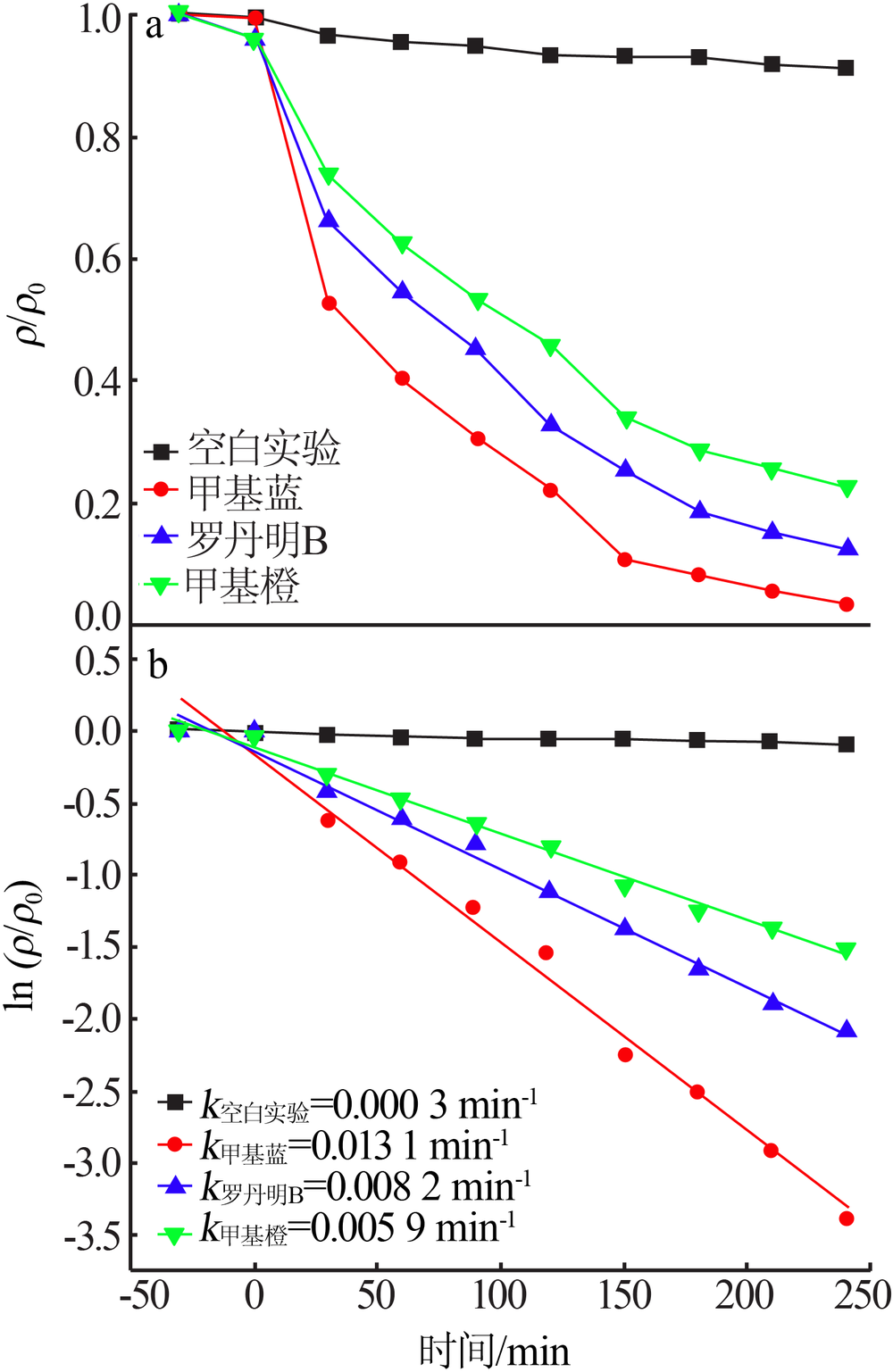
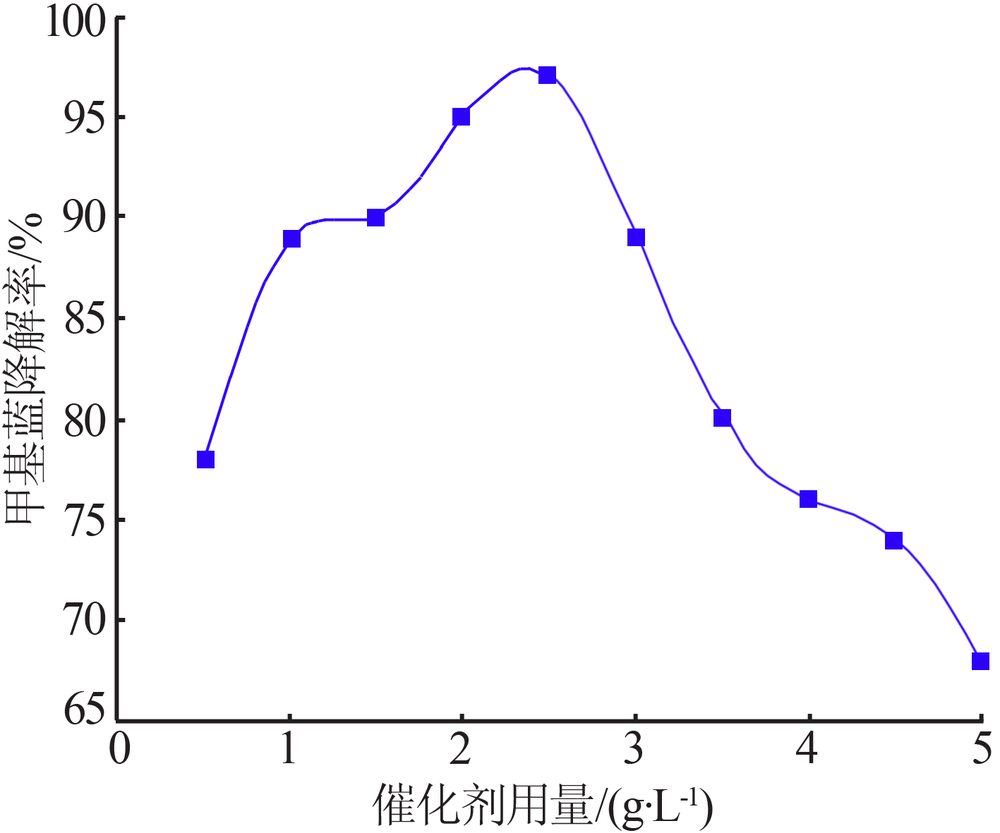
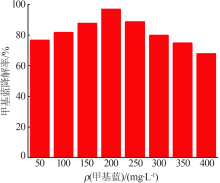
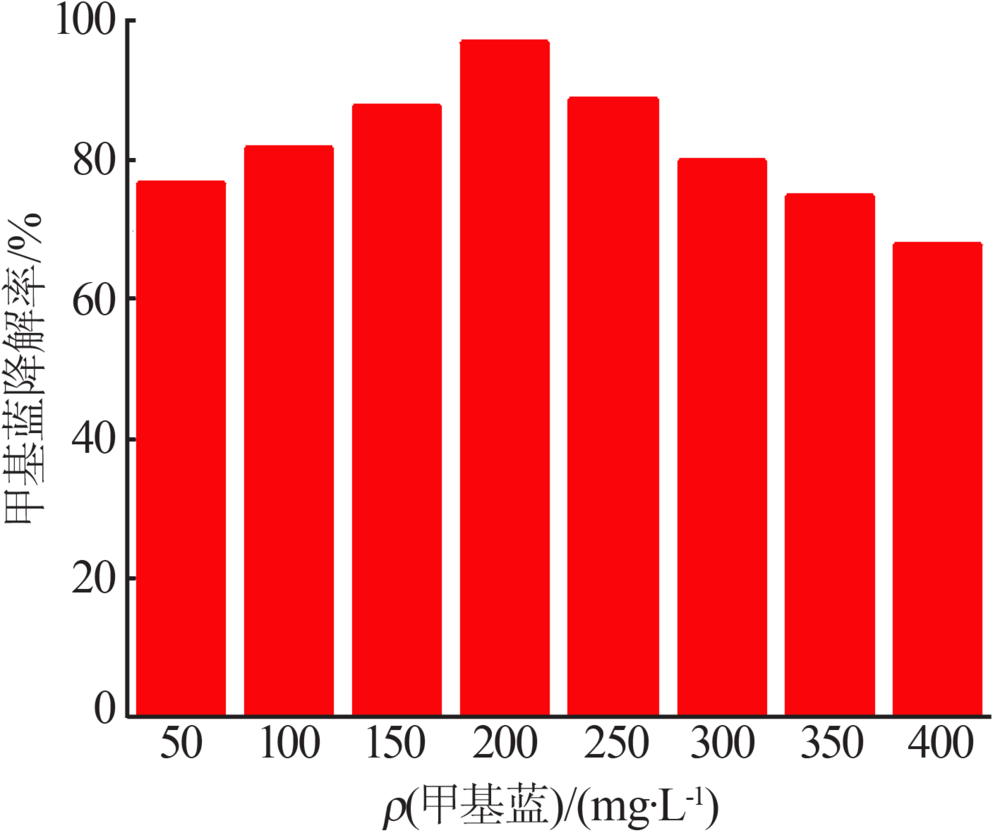
Synthesis of magnesium ferrite photocatalyst and degradation activity of various dyes
- Modern Agricultural College,Changchun Polytechnic,Jilin 130000,China
-
Received:2020-05-13Online:2020-11-10Published:2020-12-01
CLC Number:
Cite this article
Liu Ying. Synthesis of magnesium ferrite photocatalyst and degradation activity of various dyes[J]. Inorganic Chemicals Industry, 2020, 52(11): 103-107.
share this article
| [1] | 王东. 黄河流域水污染防治问题与对策[J]. 民主与科学, 2018,175(6):26-27. |
| [2] | Mills A, Davies R H, Worsley D. Water purification by semiconduc-tor photocatalysis[J]. Chem.Soc.Rev., 1993,22:417-425. |
| [3] | Hoffmann M R, Martin S T, Choi W, et al. Environmental applicati-ons of semiconductor photocatalysis[J]. Chem.Rev., 1995,95:69-96. |
| [4] | Shahid M, Jingling L, Ali Z, et al. Photocatalytic degradation of me-thylene blue on magnetically separable MgFe2O4 under visible light irradiation[J]. Mater.Chem.Phys., 2013,139:566-571. |
| [5] | Meng L, Bai H Y, Da Z L, et al. Electrospinning synjournal and pho-tocatalytic property of CaFe2O4/MgFe2O4 heterostructure for degrada-tion of tetracycline[J]. Cryst.Res.Technol., 2015,50:244-249. |
| [6] | Verma S, Joy P A, Khollam Y B, et al. Synjournal of nanosized MgFe2O4 powders by microwave hydrothermal method[J]. Mater.Lett., 2004,58(6):1092-1095. |
| [7] | Shen Y, Wu Y, Li X, et al. One-pot synjournal of MgFe2O4 nanosph-eres by solvothermal method[J]. Mater.Lett., 2013,96:85-88. |
| [8] | Li Y N, Chu Y Q, Qin Q Z. Nanocrystalline ZnFe2O4 and Ag-doped ZnFe2O4 films used as new anode materials for Li-ion batteries[J]. J.Electrochem.Soc., 2004,151:A1077-A1083. |
| [9] | 武伦鹏, 赵莲花, 张海明, 等. 光电流法研究TiO2薄膜表面吸附氧对光催化活性的影响[J]. 物理化学学报, 2007,23(5):765-768. |
| [10] | 杨晓红. MgAl2O4:Ce荧光粉辐照合成及发光机理研究[J]. 无机盐工业, 2019,51(9):30-35. |
| [11] |
Lin J, Zong R, Zhou M, et al. Photoelectric catalytic degradation of methylene blue by C60-modified TiO2 nanotube array[J]. Appl.Catal.B:Environ., 2009,89(3/4):425-431.
doi: 10.1016/j.apcatb.2008.12.025 |
| [12] | Zhu S, Xu T, Fu H, et al. Synergetic effect of Bi2WO6 photocatalyst with C60 and enhanced photoactivity under visible irradiation[J]. Environ.Sci.Technol., 2007,41(17):6234-6239. |
| [13] | 杨玉萍, 王良广, 张玉涛. 不同制备方法对氧化铁/氧化铝催化剂催化活性的影响研究[J]. 无机盐工业, 2017,49(9):78-81. |
| [14] | 沈昱, 魏浩然, 吴艳波, 等. 铁酸锰纳米球的溶剂热法制备及其可见光催化性能[J]. 大连交通大学学报, 2015,36(2):70-73. |
| [15] | 魏君, 李志奎. 多酸/二氧化钛复合光催化剂的制备及光催化苯胺的研究[J]. 分子科学学报, 2012,28(1):94-98. |
| [16] |
Konstantinou I K, Albanis T A. TiO2-assisted photocatalytic degra-dation of azo dyes in aqueous solution:kinetic and mechanistic in-vestigations[J]. Appl.Catal.B:Environ., 2004,49(1):1-14.
doi: 10.1016/j.apcatb.2003.11.010 |
| [17] | 王仕发, 杨华, 县涛, 等. 纳米YMn2O5半导体可见光催化剂[J]. 无机材料学报, 2011,26(11):1164-1168. |
| [18] | 薛红艳. 纳米铁酸钙的光催化活性与催化机理研究[J]. 无机盐工业, 2017,49(9):85-88. |
| [1] | Li Xiangguo,Guo Miao,Yao Huimin,Lü Jing. Research on hydrothermal synthesis and photoluminescence mechanism of rhombus CeO2 [J]. Inorganic Chemicals Industry, 2020, 52(6): 30-35. |
| [2] | Guo Ju,Jia Shuangzhu. Study on the one-step hydrothermal synthesis of LiFePO4 and its properties [J]. Inorganic Chemicals Industry, 2020, 52(6): 36-40. |
| [3] | An Weijia,Xie Wanli. Study on photocatalytic performance of silver carbonate [J]. Inorganic Chemicals Industry, 2020, 52(3): 107-111. |
| [4] | Zhu Jiaxin,Xiong Yuhua,Guo Rui. Research progress in modification of TiO2 photocatalyst [J]. Inorganic Chemicals Industry, 2020, 52(3): 23-27. |
| [5] | Huang Zhenxu,He Huanhuan,Jia Panpan,Chen Tiwei,Wei Shiqian. Synthesis of graphene by hydrothermal method and its electrocatalytic property on ascorbic acid [J]. Inorganic Chemicals Industry, 2020, 52(11): 29-32. |
| [6] | Chen Ping,Tian Yu,Hu Cheng. Effect of type and concentration of salt solution on synthesis of α-semi-water desulfurization gypsum [J]. Inorganic Chemicals Industry, 2020, 52(10): 130-134. |
| [7] | Wang Donghua,Fu Xin. Preparation and properties of iron oxide nanomaterials with different morphologies [J]. Inorganic Chemicals Industry, 2019, 51(9): 21-23. |
| [8] | Wu Ruifeng1,Wang Hongwei2,Pi Xiaoyuan1. Study on MoS2 composite conductive carbon as anode material forhigh performance sodium ion battery [J]. Inorganic Chemicals Industry, 2019, 51(7): 28-32. |
| [9] | Zheng Shaocong1,Zhu Liping1,Xie Gang2,Wen Zhengjiao1. Experimental study on preparation of calcium sulfate whiskers from highpurity natural gypsum with hydrothermal method [J]. Inorganic Chemicals Industry, 2019, 51(7): 39-42. |
| [10] | Zhao Yu,Zhang Shuojia,Xu Bing,Yu Yue,Sun Xiaohui. Fabrication and electrochemical properties of Zn-based electrode materials [J]. Inorganic Chemicals Industry, 2019, 51(6): 21-24. |
| [11] | Lü Pin,Shi Chunhui,Li Wei,Zhong Jianchu. Controllable synthesis and characterization of thermal stabilizer Mg-Al-LDHs [J]. Inorganic Chemicals Industry, 2019, 51(6): 29-33. |
| [12] | Mao Qiqi,Liu Chuanxin,Dong Wenjuan,Zhang Ximan,Chen Hongyu,Wu Suwen. Preparation and characterization of spheniscidite [J]. Inorganic Chemicals Industry, 2019, 51(5): 53-56. |
| [13] | Li Xin1,Yuan Bo2,Yi Meigui1. Study on removal of trace sulfur impurities from lithium carbonate by hydrothermal method [J]. Inorganic Chemicals Industry, 2019, 51(11): 28-30. |
| [14] | TIAN Xi-Qiang, ZHAO Dong-Jiang, MA Song-Yan, CHI Cai-Xia, DONG Yan-Ping, TIAN Jun, QIAO Xiu-Li. Electrocatalytic performances of NiCo2S4 compound towards oxygen reduction [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(8): 74-. |
| [15] | LI Xiao-Yun, YU Hai-Bin, SUN Yan-Min, LI Shi-Peng, ZENG Xian-Jun, SUI Yun-Le, ZHOU Peng. Inorganic aluminum salt assisted hydrothermal synthesis of pseudoboehmite by activated alumina [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(6): 35-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|