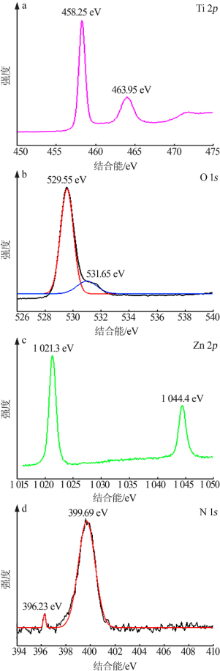
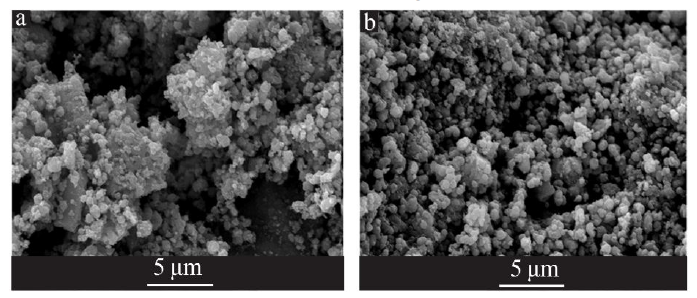
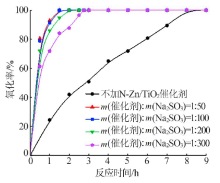
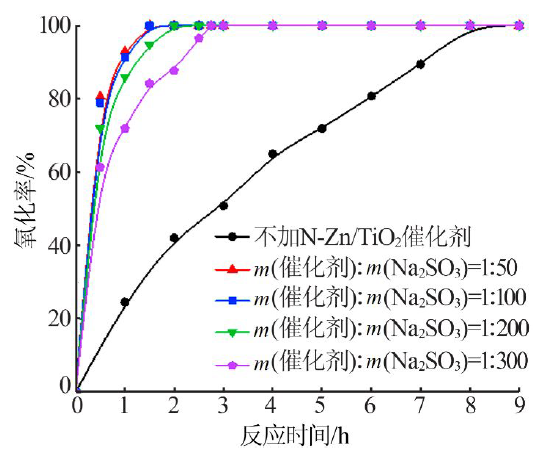
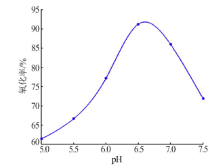
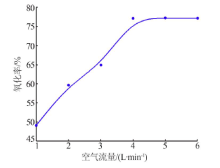
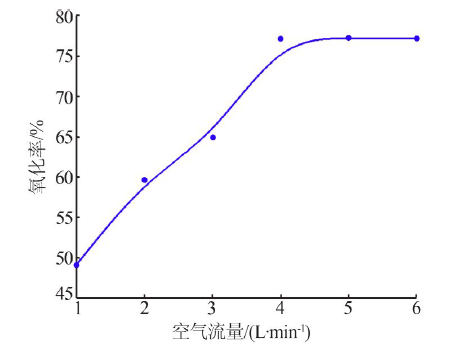
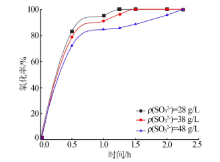
| [1] |
马连元. 双碱法脱硫技术研究[J]. 城市环境与城市生态, 2000,13(1):60-62.
|
| [2] |
马岸奇, 裘雨晓, 雷建章. 钠钙双碱法烟气脱硫技术治理方法浅析[J]. 砖瓦, 2017(8):23-27.
|
| [3] |
徐振魁. 浅谈工业烟气双碱法脱硫技术[J]. 黑龙江科技信息, 2010(8):28.
|
| [4] |
来勇. 化学沉淀—絮凝法处理双碱法烟气脱硫废水[D]. 杭州:浙江大学, 2005.
|
| [5] |
Bao J, Yang L, Sun W, et al. Removal of fine particles by heterogeneous condensation in the double-alkali desulfurization process[J]. Chemical Engineering and Processing:Process Intensification, 2011,50(8):828-835.
|
| [6] |
付新. 水热合成二氧化钛纳米颗粒及光致发光性能研究[J]. 无机盐工业, 2019,51(10):32-35.
|
| [7] |
高雅男. 二氧化钛-钒酸铋复合材料光催化降解布洛芬的研究[J]. 无机盐工业, 2019,51(6):88-91.
|
| [8] |
张少峰, 胡柏松, 陈兴林, 等. 二氧化钛纳米管阵列的制备及其强化传热应用[J]. 无机盐工业, 2018,50(1):36-40.
|
| [9] |
李丹丹, 刘中清, 颜欣. TiO2纳米管阵列光电催化氧化处理氨氮废水[J]. 无机化学学报, 2011,27(7):1358-1362.
|
| [10] |
孙勇, 刘红晶, 姚辉. 二氧化钛光催化氧化亚硫酸钠[J]. 应用化工, 2018,47(7):1373-1381.
|
| [11] |
Rao V N, Reddy N L, Kumari M M, et al. Photocatalytic recovery of H2 from H2S containing wastewater:Surface and interface control of photo-excitons in Cu2S@TiO2 core-shell nanostructures[J]. Applied Catalysis B:Environmental, 2019,254:174-185.
doi: 10.1016/j.apcatb.2019.04.090
|
| [12] |
张帅, 张立生, 李慧, 等. 二氧化钛薄膜制备工艺的研究进展[J]. 无机盐工业, 2019,51(7):15-18.
|
| [13] |
刘玲, 何玉林, 董月芬. 氮离子掺杂对二氧化钛纳米片负极材料电化学性能的影响[J]. 无机盐工业, 2018,50(10):25-28.
|
| [14] |
胡驰. 石墨烯/二氧化钛的制备及钙钛矿太阳能电池性能研究[J]. 无机盐工业, 2018,50(8):49-51.
|
| [15] |
郭婧, 戴友芝, 刘林. 光催化氧化技术在环境治理方面的研究进展[J]. 广东化工, 2019,46(16):85-86.
|
| [16] |
程萍, 顾明元, 金燕苹. TiO2光催化剂可见光化研究进展[J]. 化学进展, 2005,17(1):8-14.
|
| [17] |
Bathla A, Pal B. Bimetallic Cu(core)@Zn(shell) co-catalyst impregnated TiO2 nanosheets (001 faceted) for the selective hydrogenation of quinoline under visible light irradiation[J]. Journal of Industrial and Engineering Chemistry, 2019,79:314-325.
|
| [18] |
Xiang Jingyu, Wang Xiangdong, Zhang Kui, et al. Synjournal of N-Zn co-doped mesoporous TiO2 by a fast Sol-Gel method[J]. Rare Metal Materials and Engineering, 2016,45:51-54.
|
| [19] |
陈琦丽, 唐超群, 肖循. TiO2纳米微粒的溶胶-凝胶法制备及XRD分析[J]. 材料科学与工程, 2002,20(2):75-77.
|
| [20] |
Oladipo G O, Akinlabi A K, Alayande S O, et al. Synjournal,characterization,and photocatalytic activity of silver and zinc co-doped TiO2 nanoparticle for photodegradation of methyl orange dye in aqueous solution[J]. Canadian Journal of Chemistry, 2019,999:1-9.
|
| [21] |
罗扬. 氮掺杂与C3N4改性的TiO2光催化剂的制备及其在降解废水中的应用[D]. 广州:广州大学, 2018.
|
| [22] |
刘美琪, 陈学青, 王志彦. 铁、钴掺杂氧化锌纳米材料及其红外吸波性能研究[J]. 人工晶体学报, 2016,45(12):2785-2789.
|
| [23] |
Yao D S, Zhao Y L, Zhu L, et al. Preparation of zinc-doped titanium dioxide nanorod arrays and their application in dye sensitized solar cells[J]. International Journal of Electrochemical Science, 2015,10(7):5914-5923.
|
| [24] |
Wu C H, Kuo C Y, Lin C J, et al. Preparation of N-using a microwave/Sol-Gel method and its photocatalytic activity for bisphenol a under visible-light and sunlight irradiation[J]. International Journal of Photoenergy, 2013,10:1-9.
|
| [25] |
尚鹏博, 郑玉婴, 冀峰. 锌离子掺杂的二氧化钛介孔空心微球的制备及光催化性能[J]. 无机化学学报, 2014,30(10):2323-2331.
|
| [26] |
鞠剑峰, 缪勤华, 吴东辉. 掺氮纳米ZnO/TiO2粉体对印染废水的处理[J]. 印染, 2009,35(3):30-33.
|
| [27] |
周存, 马悦. 氮掺杂二氧化钛的制备及性能[J]. 天津工业大学学报, 2019,39(11):30-36.
|
| [28] |
柏源, 孙红旗, 金万勤. pH对氮掺杂TiO2物化性质和光催化活性的影响[J]. 无机材料学报, 2008,23(2):181-186.
|
 ),Guo Jia,Wu Huadong(
),Guo Jia,Wu Huadong( ),Zhang Linfeng
),Zhang Linfeng