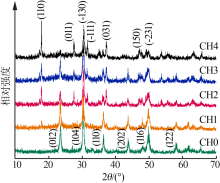
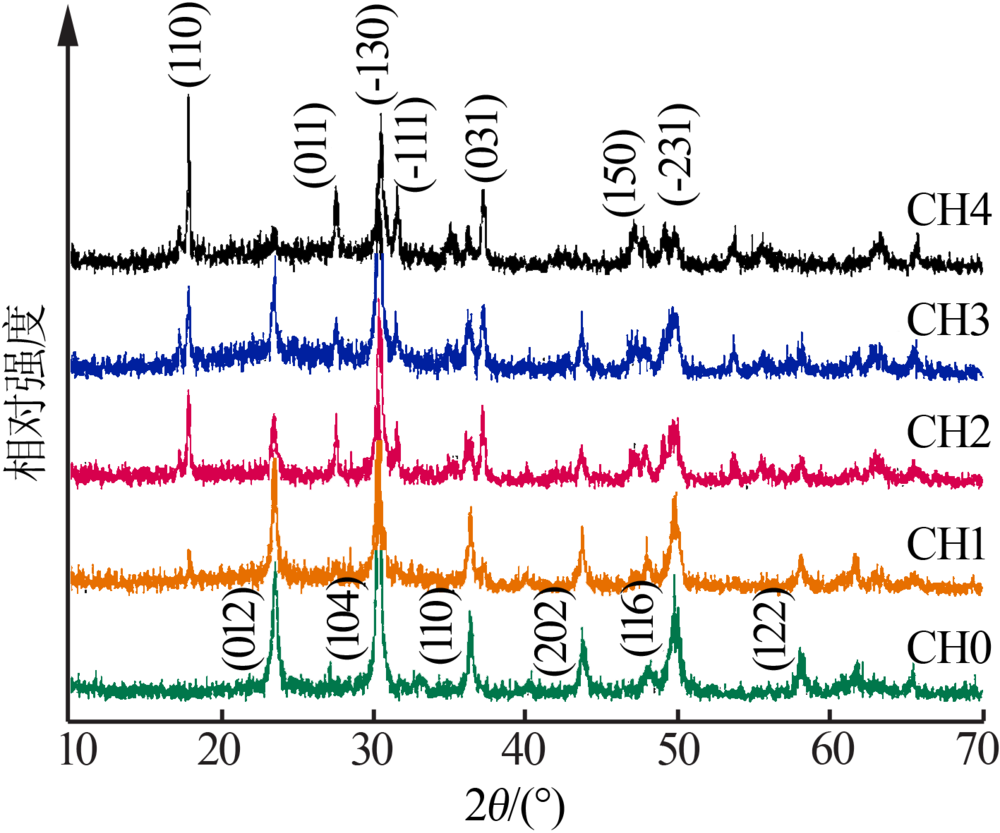
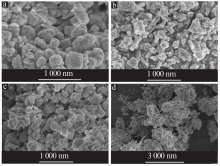
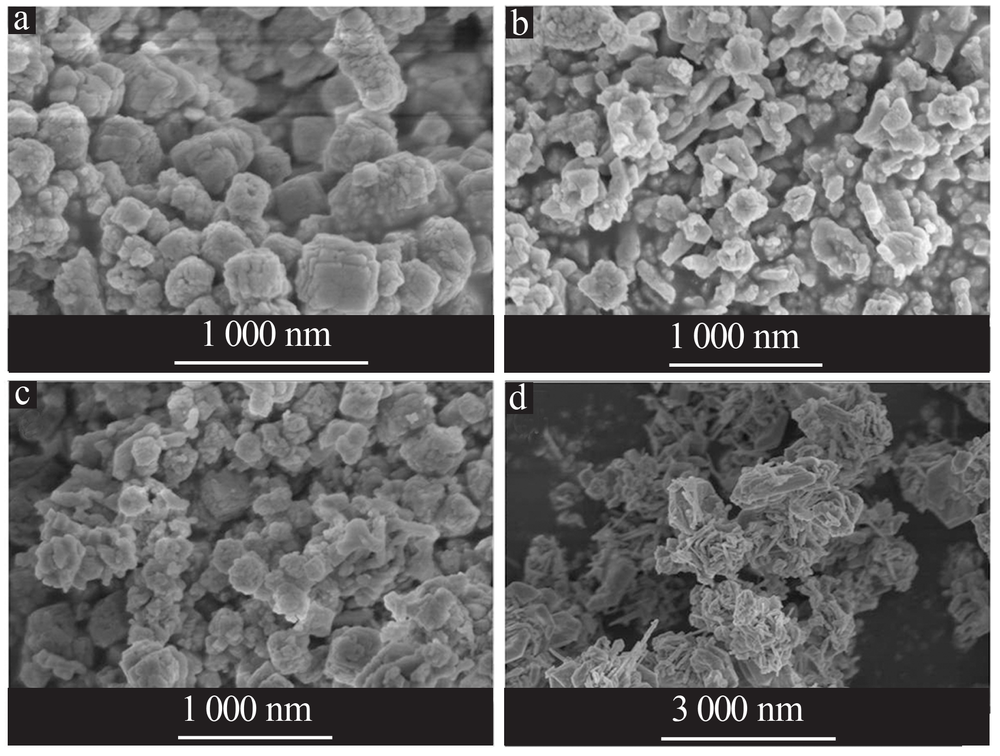
| [1] |
Cushing B L, Kolesnichenko V L, O′Connor C J, . JRecent advances in the liquid-phase syntheses of inorganic nanoparticles[J]. Chem.Rev., 2004,104:3893-3946.
doi: 10.1021/cr030027b
pmid: 15352782
|
| [2] |
Murray C B, Kagan C R, Bawendi M G . Synjournal and characteriza-tion of monodisperse nanocrystals and close-packed nanocrystalassembles[J]. Annu.Rev.Mater.Sci., 2000,30:545-610.
|
| [3] |
Rogach A L . Semiconductor nanocrystal quantum dots:synbook,assembly,spectroscopy and applications[M]. New York:Springer Wien, 2008.
|
| [4] |
Moon G D, Ko S, Min Y , et al. Chemical transformations of nanostuc-tured materials[J]. Nano Today, 2011,6(2):186-203.
doi: 10.1016/j.nantod.2011.02.006
|
| [5] |
Moon G D, Ko S, Xia Y , et al. Chemical transformations in ultrathin chalcogenide nanowires[J]. ACS Nano, 2010,4(4):2307-2319.
doi: 10.1021/nn9018575
pmid: 20337466
|
| [6] |
Park J, Zheng H, Jun Y W , et al. Hetero-epitaxial anion exchange yields single-crystalline hollow nanoparticles[J]. J.Am.Chem.Soc., 2009,131(39):13943-13946.
doi: 10.1021/ja905732q
pmid: 19788329
|
| [7] |
Beberwyck B J, Surendranath Y, Alivisatos A P . Cation exchange:a versatile tool for nanomaterials synjournal[J]. J.Phys.Chem.C, 2013,117:19759-19770.
|
| [8] |
Klinkova A . Cation exchange reactions in semiconductor nanocryst-als[D].Kentucky:Graduate College of Bowling Green State Univer-sity, 2011.
|
| [9] |
Tan Z, Sun Z H, Guo Q , et al. A novel ion-exchange method for the synjournal of nano-SnO/micro-C hybrid structure as high capacity anode material in lithium ion batteries[J]. J.Mater.Sci.Technol., 2013,29(7):609-612.
doi: 10.1007/s11259-005-3382-x
pmid: 16142608
|
| [10] |
Fayette M, Robinson R D . Chemical transformations of nanomateri-als for energy applications[J]. J.Mater.Chem.A, 2014,2:5965-5978.
|
| [11] |
Yan C L, Rosei F . Hollow micro/nanostructured materials prepared by ion exchange synjournal and their potential applications[J]. New J.Chem., 2014,38(5):1883-1904.
|
| [12] |
逯亚飞, 孔祥荣, 胡鹏 , 等. Cd(OH)2纳米材料制备研究进展[J]. 现代化工, 2014,34(8):27-31.
|
| [13] |
刘俊渤, 常海波, 窦森 , 等. 水热合成法制备六方形纳米盘Cd(OH)2[J]. 吉林大学学报:理学版, 2009,47(3):605-608.
|
| [14] |
Chen Q C, Wu Q S . Synjournal and photocatalytic property of Cd(OH)2 nanowires based on a supported liquid membrane system[J]. Mi-cro & Nano Letters, 2011,6(3):150-153.
|
| [15] |
Tao Yugui, Wan Yong, Zheng Shuxiang , et al. Phenolic resin assisted synjournal of SiO2/TiO2 mesoporous microspheres and their photoca-talytic performance[J]. Fine Chemicals, 2015,32(5):491-495.
|
| [16] |
Tao Tingxian, Xu Jiabin, Chu Wei . Synjournal and photocatalytic properties of nano-Bi2MoO6/amidoxime fiber composites[J]. Fine Chemicals, 2016,33(3):320-325.
|