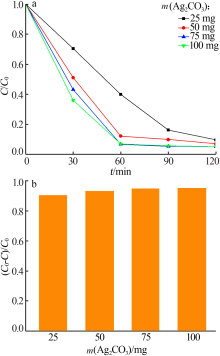
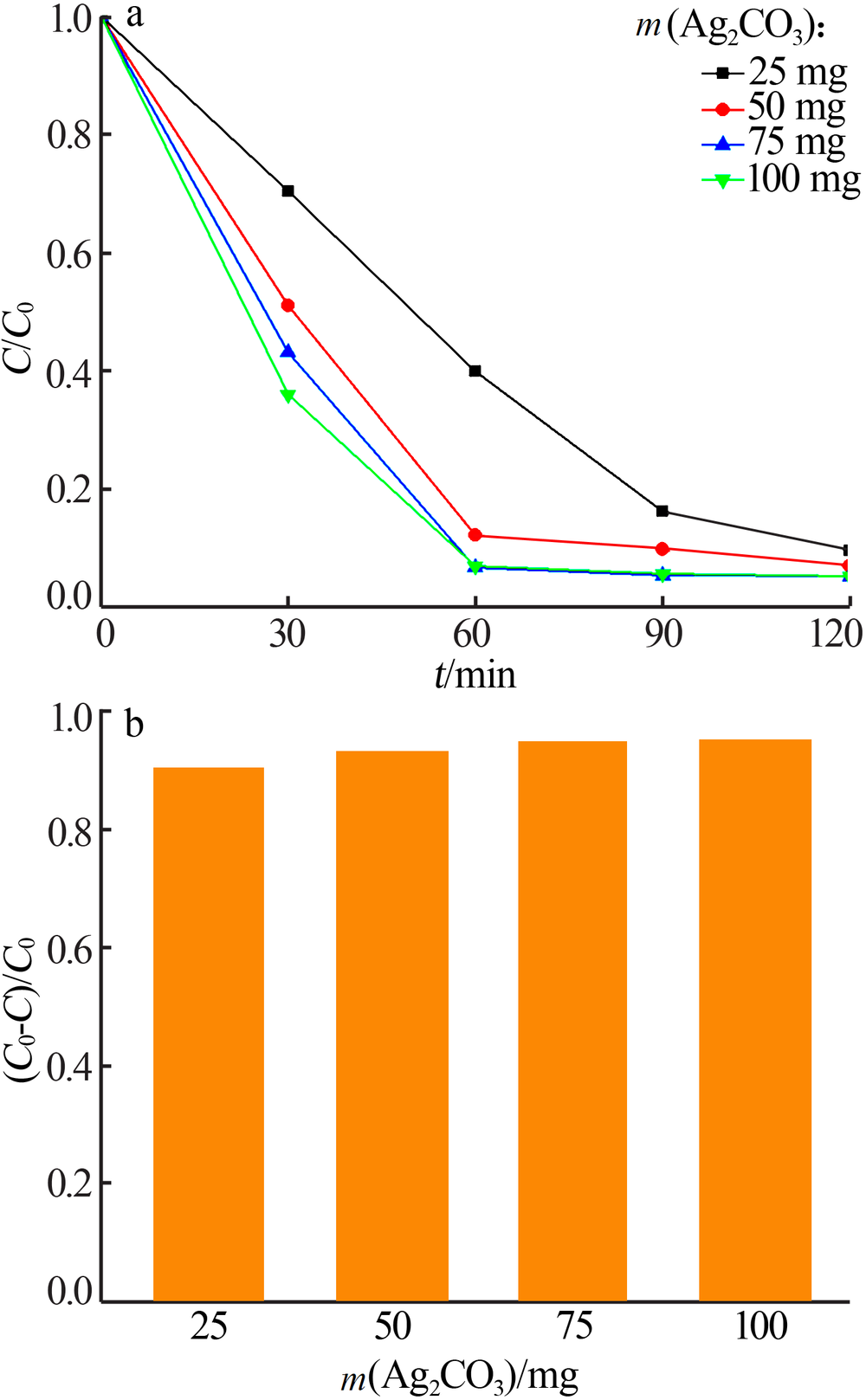
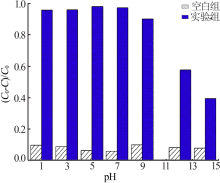
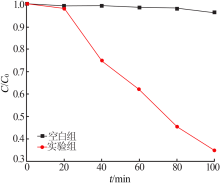
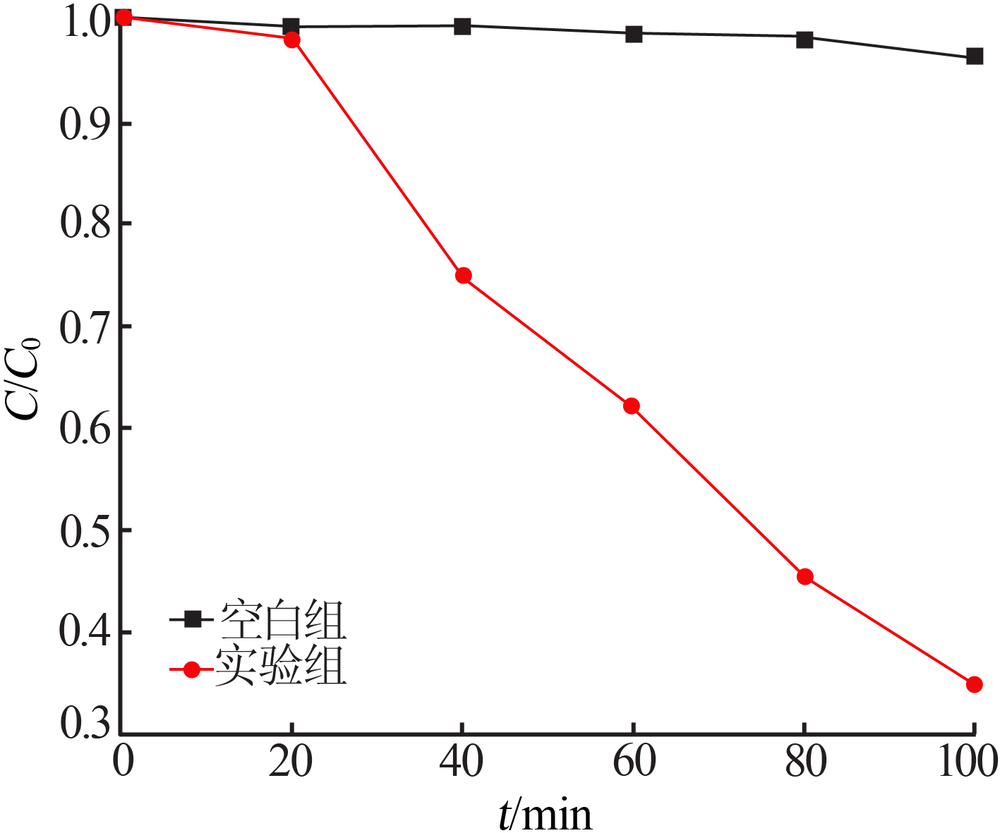
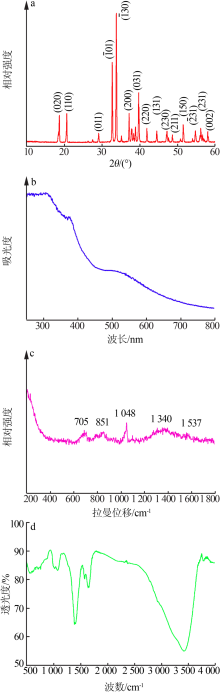
Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (3): 107-111.doi: 10.11962/1006-4990.2019-0251
• Catalytic Materials • Previous Articles
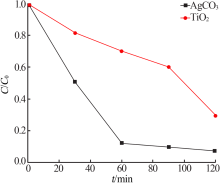
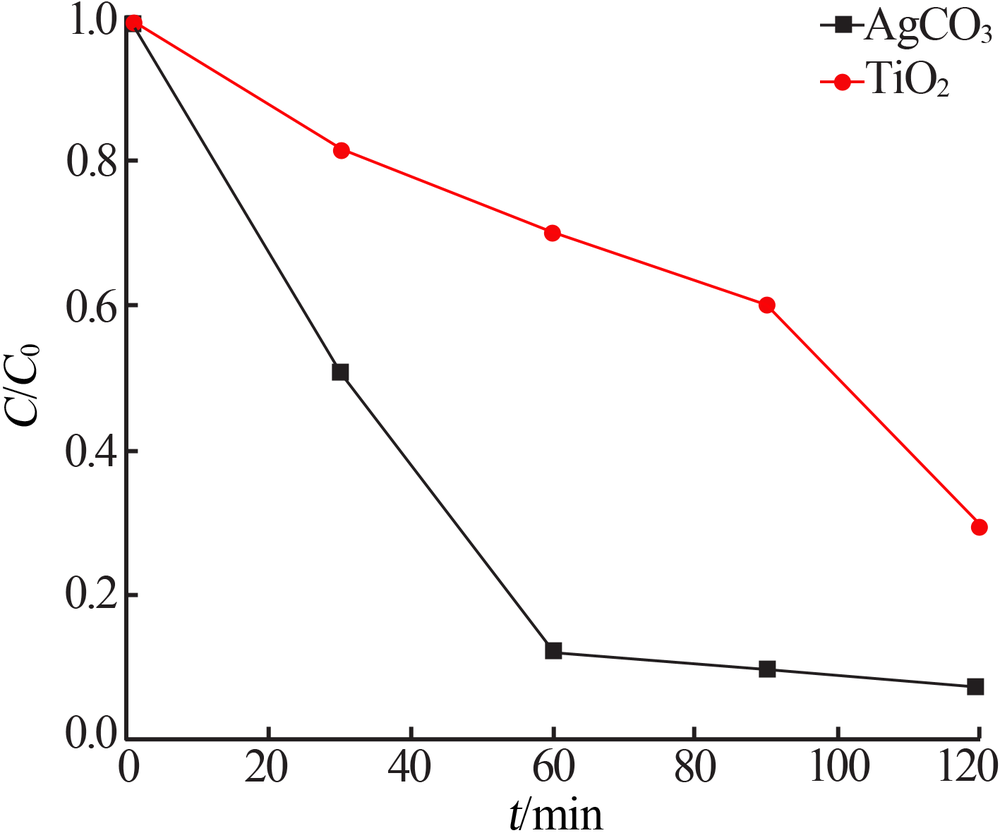
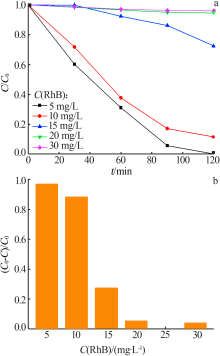
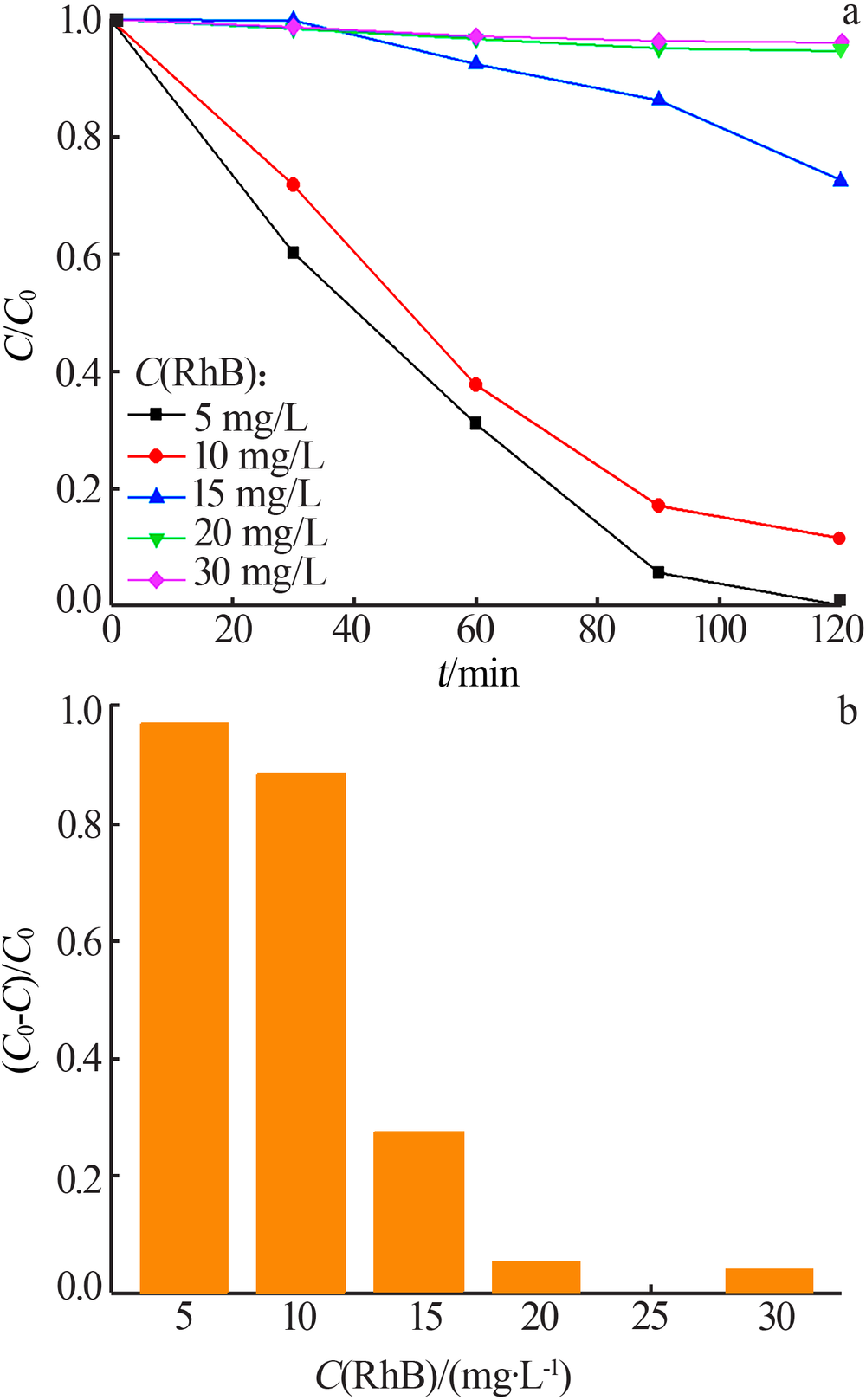
Study on photocatalytic performance of silver carbonate
An Weijia,Xie Wanli
- North China University of Science and Technology,Tangshan 063210,China
-
Received:2019-09-13Online:2020-03-10Published:2020-03-31
CLC Number:
Cite this article
An Weijia,Xie Wanli. Study on photocatalytic performance of silver carbonate[J]. Inorganic Chemicals Industry, 2020, 52(3): 107-111.
share this article
| [1] | 李金环 . 掺杂TiO2光催化剂的制备、表征及降解含聚污水研究[D]. 大庆:大庆石油学院, 2008. |
| [2] | 曹玉辉 . 具有{101}与{001}晶面异质结TiO2纳米晶的制备及其光催化还原CO2性能研究[D]. 开封:河南大学, 2016. |
| [3] | Dai G, Yu J, Liu G . A new approach for photocorrosion inhibition ofAg2CO3 photocatalyst with highly visible-light-responsive reactivi-ty[J]. The Journal of Physical Chemistry C, 2012,116(29):15519-15524. |
| [4] | Kubacka A, Fernandez-Garcia M, Colon G . Advanced nanoarchitec-tures for solar photocatalytic applications[J]. Chemical Reviews, 2011,112(3):1555-1614. |
| [5] | Tong H, Ouyang S, Bi Y , et al. Nano-photocatalytic materials:possi-bilities and challenges[J]. Advanced Materials, 2012,24(2):229-251. |
| [6] | Yi Z, Ye J, Kikugawa N , et al. An orthophosphate semiconductorwith photooxidation properties under visible-light irradiation[J]. Nature Materials, 2010,9(7):559-564. |
| [7] | Li J, Wei L, Yu C , et al. Preparation and characterization of grapheneoxide/Ag2CO3 photocatalyst and its visible light photocatalytic acti-vity[J]. Applied Surface Science, 2015,358:168-174. |
| [8] | 王瑜琦 . 碳酸银基半导体复合材料的制备及其光催化性能研究[D]. 镇江:江苏大学, 2016. |
| [9] | Varma R, Yadav M, Tiwari K , et al. Roles of vanadium and nitrogenin photocatalytic activity of VN-codoped TiO2 photocatalyst[J]. Photochemistry and Photobiology, 2018,94(5):955-964. |
| [10] | 王震 . 新型可见光催化剂的制备及其性能研究[D]. 杭州:浙江大学, 2014. |
| [11] | 邱毅伟 . 基于Ag3PO4纳米复合材料的设计、制备和光催化性能研究[D]. 杭州:浙江理工大学, 2016. |
| [12] | 闫世成, 罗文俊, 李朝升 , 等. 新型光催化材料探索和研究进展[J]. 中国材料进展, 2010,29(1):1-9,53. |
| [13] | Ng Y H, Iwase A, Kudo A , et al. Reducing graphene oxide on a visi-ble-light BiVO4 photocatalyst for an enhanced photoelectrochemi-cal water splitting[J]. The Journal of Physical Chemistry Letters, 2010,1(17):2607-2612. |
| [14] | Wu L, Bi J, Li Z , et al. Rapid preparation of Bi2WO6 photocatalystwith nanosheet morphology via microwave-assisted solvothermal synjournal[J]. Catalysis Today, 2008,131(1/2/3/4):15-20. |
| [15] | Wen J, Xie J, Chen X , et al. A review on g-C3N4-based photocata-lysts[J]. Applied Surface Science, 2017,391:72-123. |
| [16] | Li Q, Li X, Wageh S , et al. CdS/graphene nanocomposite photocat-alysts[J]. Advanced Energy Materials, 2015,5(14):1500010. |
| [17] | Ha E, Lee L Y S, Wang J , et al. Significant enhancement in photo-catalytic reduction of water to hydrogen by Au/Cu2ZnSnS4 nanos-tructure[J]. Advanced Materials, 2014,26(21):3496-3500. |
| [18] | 林婵 . TiO2纳米材料的制备、改性及其光催化性能研究[D]. 青岛:中国海洋大学, 2014. |
| [19] | Wen X J, Niu C G, Guo H , et al. Photocatalytic degradation of levo-floxacin by ternary Ag2CO3/CeO2/AgBr photocatalyst under visible-light irradiation:Degradation pathways,mineralization ability,and an accelerated interfacial charge transfer process study[J]. Journal of Catalysis, 2018,358:211-223. |
| [20] | Hu W, Zhao L, Zhang Y , et al. Preparation and photocatalytic acti-vity of graphene-modified Ag2S composite[J]. Journal of Experi-mental Nanoscience, 2016,11(6):433-444. |
| [21] | Frontistis Z, Antonopoulou M, Petala A , et al. Photodegradation ofethyl paraben using simulated solar radiation and Ag3PO4 photo-catalyst[J]. Journal of Hazardous Materials, 2017,323:478-488. |
| [22] | Zhu T, Song Y, Ji H , et al. Synjournal of g-C3N4/Ag3VO4 compositeswith enhanced photocatalytic activity under visible light irradiation[J]. Chemical Engineering Journal, 2015,271:96-105. |
| [23] | 周丽, 邓慧萍, 张为 . 可见光响应的银系光催化材料[J]. 化学进展, 2015,27(4):349-360. |
| [24] | 任南琪, 周显娇, 郭婉茜 , 等. 染料废水处理技术研究进展[J]. 化工学报, 2013,64(1):84-94. |
| [25] | Xu C, Liu Y, Huang B , et al. Preparation,characterization,and pho-tocatalytic properties of silver carbonate[J]. Applied Surface Sci-ence, 2011,257(20):8732-8736. |
| [26] | 张嘉慧 . β-Ag2CO3纳米粒子的制备及催化端炔氢叠氮化反应研究[D]. 长春:东北师范大学, 2018. |
| [27] | 魏龙福, 余长林, 陈建钗 , 等. 水热法合成Ag2CO3/ZnO异质结复合光催化剂及其光催化性能[J]. 有色金属科学与工程, 2014,5(1):47-53. |
| [28] | Dong H, Chen G, Sun J , et al. A novel high-efficiency visible-lightsensitive Ag2CO3 photocatalyst with universal photodegradationperformances:simple synjournal,reaction mechanism and first-pr-inciples study[J]. Applied Catalysis B:Environmental, 2013,134:46-54. |
| [29] | 余忠雄, 向垒, 钟方龙 , 等. pH对低温燃烧法合成钨酸铋光催化降解罗丹明B的影响[J]. 无机材料学报, 2015,30(5):535-541. |
| [30] | 郝辰春, 贾莹, 王文忠 . Ag2CO3/Ag2O异质p-n结光催化剂的制备及其可见光光催化性能[J]. 中央民族大学学报:自然科学版, 2015,24(2):76-81. |
| [31] | Song Y, Zhu J, Xu H , et al. Synjournal,characterization and visible-light photocatalytic performance of Ag2CO3 modified by graphene-oxide[J]. Journal of Alloys and Compounds, 2014,592:258-265. |
| [32] | Yu C, Wei L, Zhou W , et al. A visible-light-driven core-shell likeAg2S@Ag2CO3 composite photocatalyst with high performance inpollutants degradation[J]. Chemosphere, 2016,157:250-261. |
| [1] | Zhou Jian,Tang Hongbo. Preparation and photocatalytic properties of ZnWO4/Ag3PO4 composite photocatalyst [J]. Inorganic Chemicals Industry, 2020, 52(6): 101-104. |
| [2] | Zhu Jiaxin,Xiong Yuhua,Guo Rui. Research progress in modification of TiO2 photocatalyst [J]. Inorganic Chemicals Industry, 2020, 52(3): 23-27. |
| [3] | Liu Ying. Synthesis of magnesium ferrite photocatalyst and degradation activity of various dyes [J]. Inorganic Chemicals Industry, 2020, 52(11): 103-107. |
| [4] | Huang Lili,Zhao Shenmao,Deng Yuequan. Preparation of rare earth Nd-doped ZnO nano-materials and its degradation of Rhodamine B [J]. Inorganic Chemicals Industry, 2019, 51(8): 88-92. |
| [5] | FAN Xue-Min, BAI Chun-Hua, LI Guang-Hui, XU Zhi-Yong, SUN Chun-Bao. Research progress in co-doping modification of nano TiO2 photocatalysts [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(10): 7-. |
| [6] | CHEN Xiao-Xia, MA Xue-Lian, GUO Gui-Bao, GUO Xiao-Hui. Preparation and photocatalysis performance of strontium borate nano-powders by carbon adsorption [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(1): 75-. |
| [7] | . Preparation and photocatalytic activity of tungsten oxide hollow sphere [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(6): 79-. |
| [8] | LIU Li, WANG Ya-Fei, CUI Wen-Quan, LIANG Ying-Hua, WANG Meng. Preparation of BiVO4 and photocatalytic degradation of RhB under visible light [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(8): 60-. |
| [9] | CUI Wen-Quan, AN Wei-Jia, LIU Li, QI Yue-Li, LIANG Ying-Hua, ZHANG Ting. Preparation of CdS-pillared K2La2Ti3-xNbxO10 composite and photocatalytic properties thereof [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(8): 56-. |
| [10] | MAO Zhi-Qiang, LENG Yuan-Peng, ZHAO Ya-Ping. Continuous preparation of nano-sized zinc oxide by supercritical water technique and photocatalytic activity thereof [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(10): 53-. |
| [11] | WANG Yu-Guang. Present research status of nano-sized TiO2 photocatalyst [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(3): 50-. |
| [12] | TIAN Yong-Shu, MA Si-Yuan, HOU Run-Xin. Study on preparation of multi-element modified titanium dioxide and performances thereof [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(11): 30-. |
| [13] | LIU Zi-Quan, JIANG Fu-Yi, MA Xia, LIU Rui-Cui, JIANG Run-Qian. Research progress in supported materials and loading method of photocatalyst [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(11): 6-. |
| [14] | Zhang Guoyi;Li Ling. Determination of gallium trioxide in red mud by extraction-photometric method with butylrhodamine B [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(9): 0-0. |
| [15] | Wang Cheng;Shi Huisheng;Zhang Pu;Li Yan. Effect of heat treatment temperature on photocatalytic activity of natural zinc blende [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(7): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|