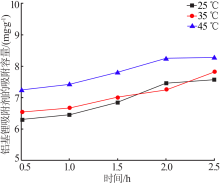
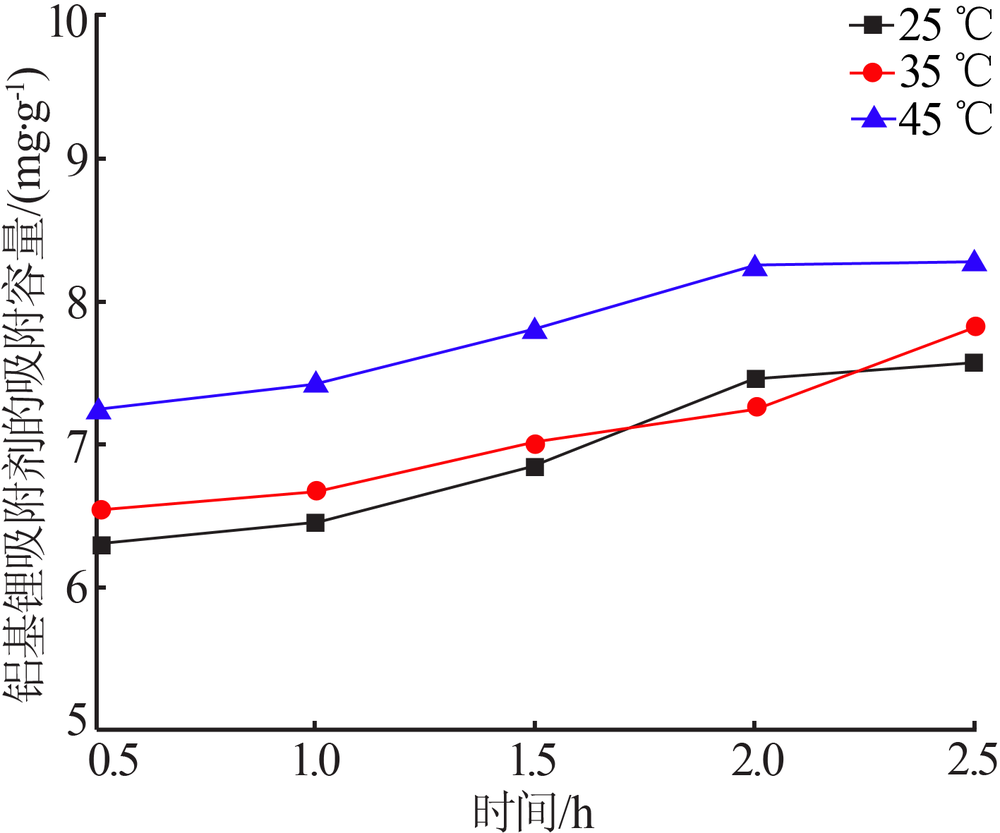
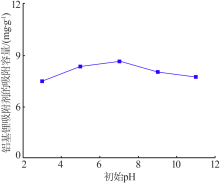
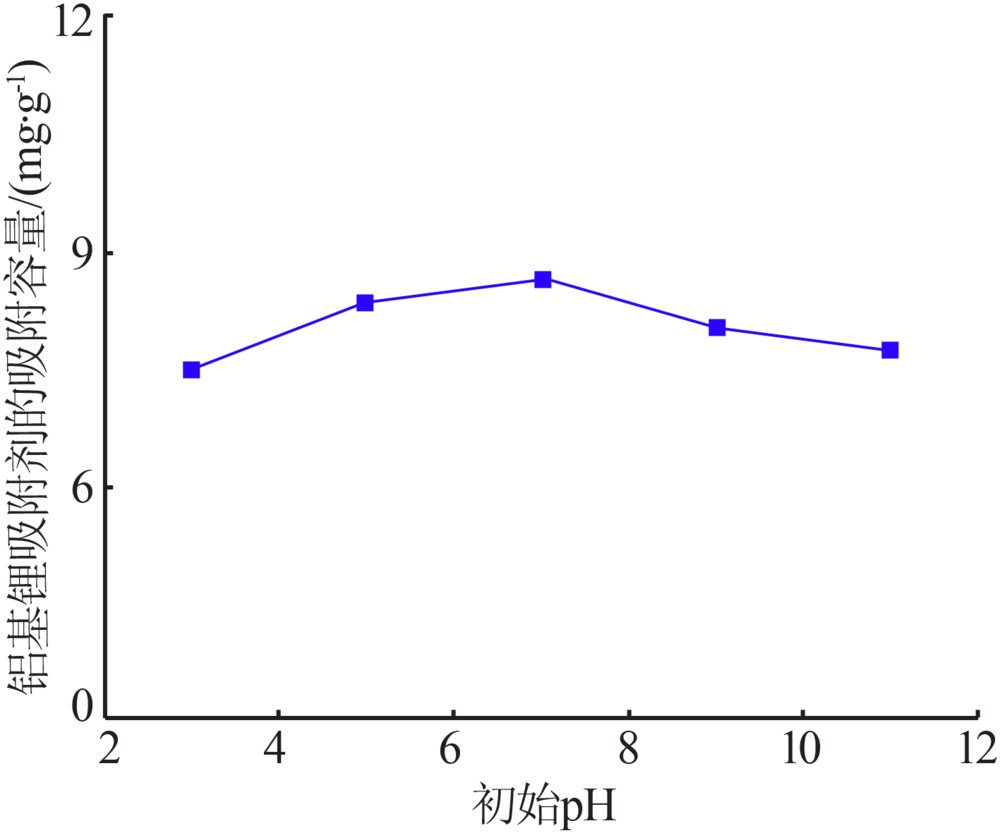
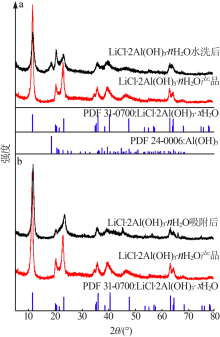
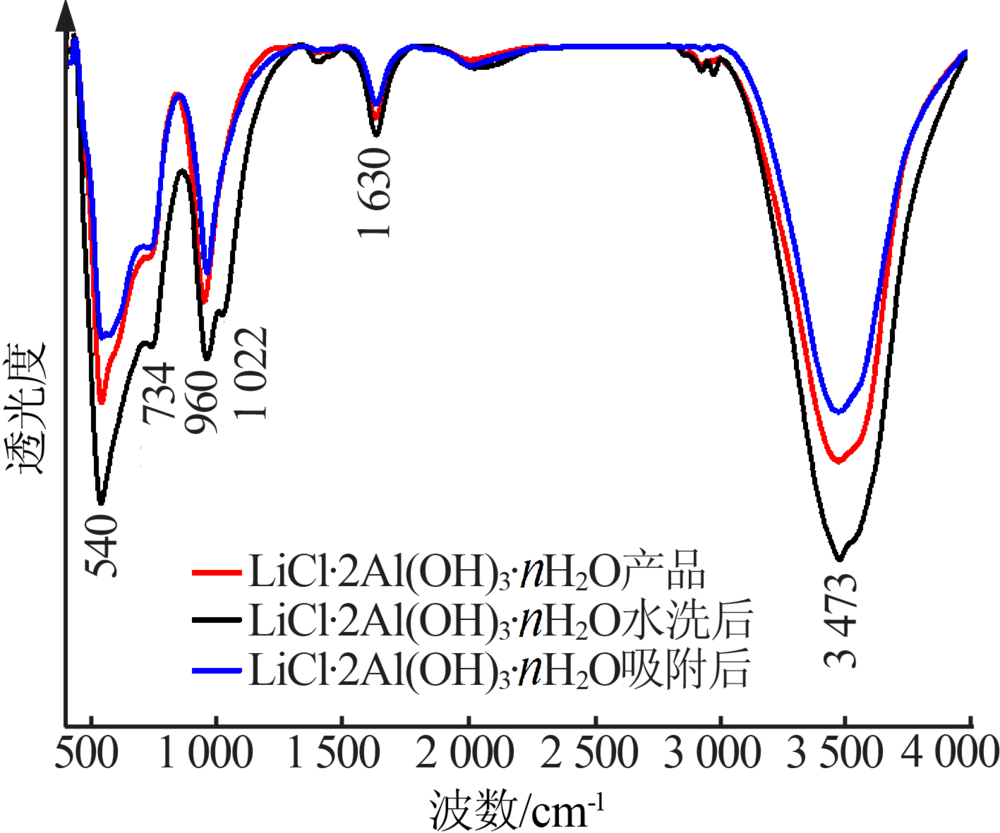
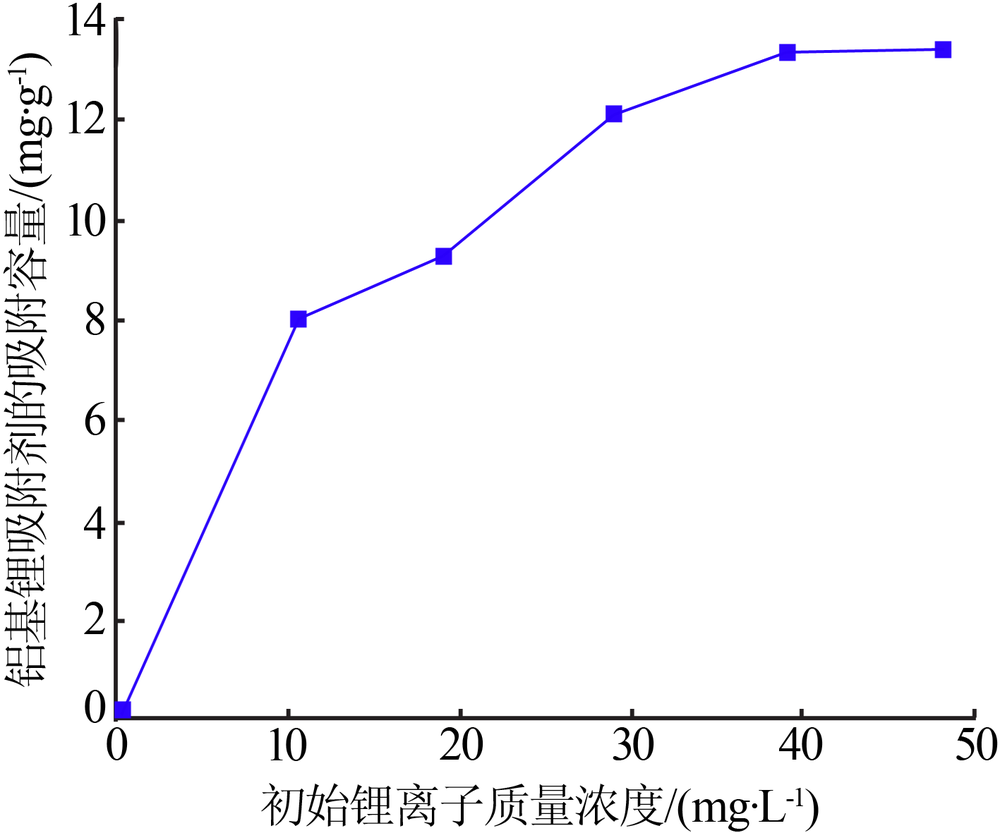
Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (8): 51-56.doi: 10.11962/1006-4990.2019-0499
• Research & Development • Previous Articles Next Articles
Preparation and adsorption properties of aluminum-based lithium adsorbent
Tang Na,Gong Jingkuan,Xiang Jun( )
)
- College of Chemical Engineering and Material Science,Tianjin University of Science and Technology,Tianjin 300457,China
-
Received:2020-02-27Online:2020-08-10Published:2020-08-12 -
Contact:Xiang Jun E-mail:jxiang@tust.edu.cn
CLC Number:
Cite this article
Tang Na,Gong Jingkuan,Xiang Jun. Preparation and adsorption properties of aluminum-based lithium adsorbent[J]. Inorganic Chemicals Industry, 2020, 52(8): 51-56.
share this article
"
| 水平 | 因素 | 吸附量/ (mg·g-1) | ||||
|---|---|---|---|---|---|---|
| A | 空 | B | C | D | ||
| 1 | 1 | 1 | 1 | 1 | 1 | 6.14 |
| 2 | 1 | 2 | 2 | 2 | 2 | 6.82 |
| 3 | 1 | 3 | 3 | 3 | 3 | 6.32 |
| 4 | 1 | 4 | 4 | 4 | 4 | 6.65 |
| 5 | 2 | 1 | 2 | 4 | 4 | 7.30 |
| 6 | 2 | 2 | 1 | 3 | 3 | 6.20 |
| 7 | 2 | 3 | 4 | 1 | 2 | 6.66 |
| 8 | 2 | 4 | 3 | 2 | 1 | 6.43 |
| 9 | 3 | 1 | 3 | 4 | 2 | 7.27 |
| 10 | 3 | 2 | 4 | 3 | 1 | 7.41 |
| 11 | 3 | 3 | 1 | 2 | 4 | 7.49 |
| 12 | 3 | 4 | 2 | 1 | 3 | 8.18 |
| 13 | 4 | 1 | 4 | 2 | 3 | 6.43 |
| 14 | 4 | 2 | 3 | 1 | 4 | 4.99 |
| 15 | 4 | 3 | 2 | 4 | 1 | 8.21 |
| 16 | 4 | 4 | 1 | 3 | 2 | 7.90 |
| K1 | 6.48 | 6.78 | 6.91 | 6.49 | 7.05 | |
| K2 | 6.65 | 6.35 | 7.63 | 6.80 | 7.16 | |
| K3 | 7.59 | 7.17 | 6.25 | 7.23 | 6.78 | |
| K4 | 6.88 | 7.29 | 6.80 | 7.07 | 6.61 | |
| R | 4.42 | 3.75 | 5.49 | 2.97 | 2.22 | |
| [1] | 刘东帆, 孙淑英, 于建国, 等. 盐湖卤水提锂技术研究与发展[J]. 化工学报, 2018,69(1):141-155. |
| [2] | 高峰, 郑绵平, 乜贞, 等. 盐湖卤水锂资源及其开发进展[J]. 地球学报, 2011,32(4):483-492. |
| [3] | 肖小玲, 戴志锋, 祝增虎, 等. 吸附法盐湖卤水提锂的研究进展[J]. 盐湖研究, 2005,13(2):66-69. |
| [4] | 董茜, 李燕杰, 朴香兰, 等. 铝盐吸附剂从盐湖卤水中吸附锂的研究[J]. 稀有金属, 2007,31(3):357-361. |
| [5] | 吴志坚, 郭敏, 李权, 等. 氢氧化铝基锂吸附剂从卤水中吸附锂的机理[J]: 盐湖研究, 2018,26(3):1-6. |
| [6] | 罗清平, 郭朋成, 杨志平, 等. 用AI-9吸附剂从盐湖卤水中吸附锂的实验研究[J]. 湿法冶金, 2012,31(3):152-154. |
| [7] | 郭敏. 一种微孔铝盐锂吸附剂及其制备方法、填料和富集锂离子的方法:中国,106076243A[P]. 2016-11-09. |
| [8] | 郭敏, 刘忠, 李权, 等. 铝基锂吸附剂从卤水中吸附提锂的研究及进展[J]. 青海科技, 2019,26(3):16-20. |
| [9] | Bauman W C. Composition for the recovery of lithium values from brine and process of making/using said composition:US,6280693[P]. 2001-08-28. |
| [10] | 李杰, 熊小波. 铝盐吸附剂盐湖卤水提锂的研究现状及展望[J]. 无机盐工业, 2010,42(10):9-11. |
| [1] | Lu Yuyuan,Xu Song,Su Tong,Yang Zhibin,Zhang Qiqin,Zhang Yang. Study on physical and chemical characteristics and adsorption performance of converter desulfurization slag [J]. Inorganic Chemicals Industry, 2021, 53(1): 87-90. |
| [2] | Wang Kuangbin,Xu Shengxia,Wang Yongqin. Research on purification process of lithium difluorosulfimide optimized by response surface method [J]. Inorganic Chemicals Industry, 2020, 52(9): 62-65. |
| [3] | Zhao Chuang,Fan Jingxin,Guo Chunlei,Li Ben,Li Bin,Zang Jiazhong,Wang Yang,Hou Liwei,Sun Zhenhai. Study on deactivation of diesel aromatic adsorbent [J]. Inorganic Chemicals Industry, 2020, 52(8): 98-102. |
| [4] | Yan Yajing. Research status and prospect of solid electrolyte for lithium ion batteries [J]. Inorganic Chemicals Industry, 2020, 52(7): 22-25. |
| [5] | Wang Jiatai,Zhao Duan,Ma Lianhua,Zhang Caihong. Research progress of LiFePO4 cathode materials for Li-ion battery [J]. Inorganic Chemicals Industry, 2020, 52(4): 18-22. |
| [6] | Xu Naicai,Li Sixia,Cao Jiajia,Yang Gangshenga. Research on doping modification and adsorption performance of manganese oxides lithium ion sieve [J]. Inorganic Chemicals Industry, 2020, 52(4): 37-41. |
| [7] | Zhang Wending,Deng Xiaochuan,Zhu Chaoliang,Fan Faying,Zhang Yi,Qing Binju. Preparation of lithium adsorbent from PAC and its adsorption properties [J]. Inorganic Chemicals Industry, 2020, 52(2): 12-16. |
| [8] | Bian Xiaotong,Qiu Zhaofu,Yang Ji,Yan Ruiqi,Lü Shuguang. Study on fluoride removal by modified Y zeolite particles with sodium alginate-lanthanum hydroxide [J]. Inorganic Chemicals Industry, 2020, 52(12): 86-91. |
| [9] | Deng Kui,Pei Guangbin,Wang Qian. Research on preparation process of super-fine aluminum hydroxide with low crystal seed ratio [J]. Inorganic Chemicals Industry, 2020, 52(10): 121-124. |
| [10] | Luo Chengguo,Xiao Jun,Fan Guangxin. Current situation and development of precursors for LiMn2O4 cathode used in lithium ion batteries [J]. Inorganic Chemicals Industry, 2020, 52(1): 26-29. |
| [11] | Liu Bingguang,Zu Xiaodong,Li Jiansheng,Lu Junfeng,Wang Zejiang. Research progress in supported lithium ion sieve absorbents [J]. Inorganic Chemicals Industry, 2019, 51(9): 12-16. |
| [12] | Zhang Bin,Han Yuxia,Zhao Siqin, S.Asuha. Preparation of γ-Fe2O3 nanoparticles and its adsorption properties [J]. Inorganic Chemicals Industry, 2019, 51(6): 76-79. |
| [13] | Sun Peiliang,Chen Shijuan,Yuan Li. Preparation and electrochemical properties of lithium difluorophosphate [J]. Inorganic Chemicals Industry, 2019, 51(5): 45-48. |
| [14] | Ye Liuying,Zeng Dewen,Chen Chi,Li Zhuye,Li Gao,Liao Yipeng,Xu Baoming. Research progress in application of absorbent of lithium extraction from brine [J]. Inorganic Chemicals Industry, 2019, 51(3): 16-19. |
| [15] | Yu Xinrui,Gao Feng,Lü Chao,Sheng Yu,Tong Qingsong. Effects of aging temperature on electrochemical properties of LiNi0.5Co0.2Mn0.3O2 cathode material [J]. Inorganic Chemicals Industry, 2019, 51(10): 51-55. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|