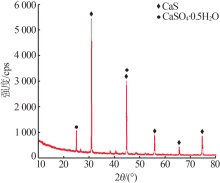
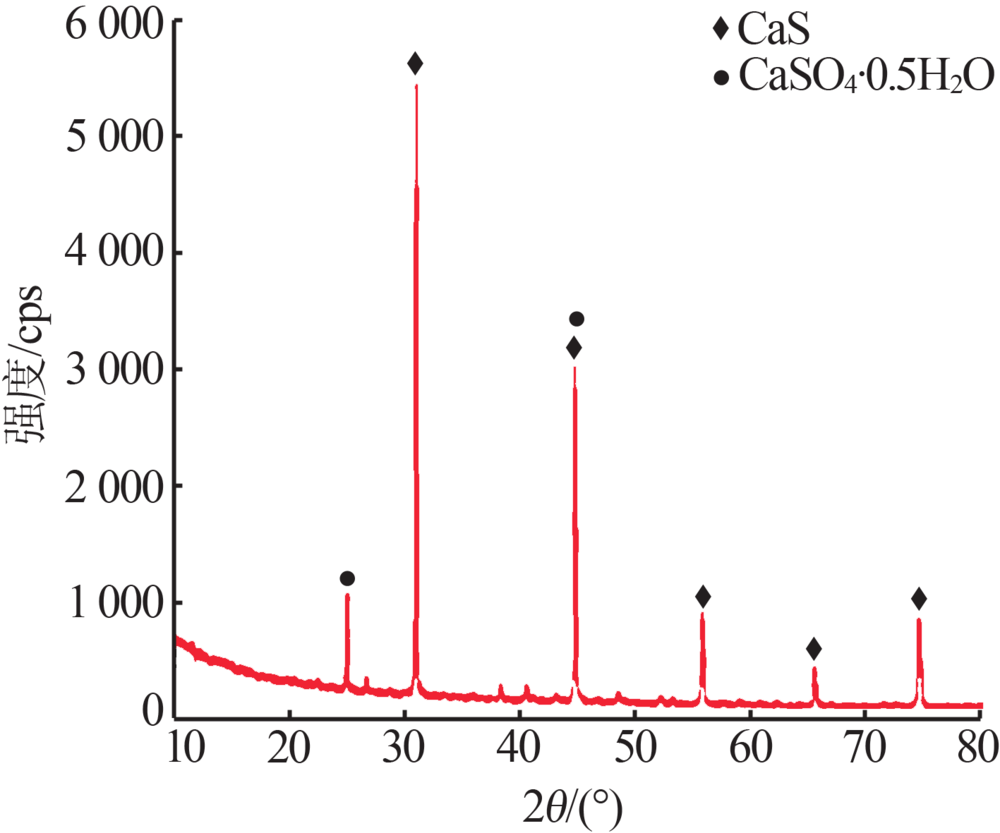
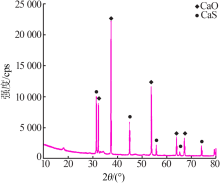
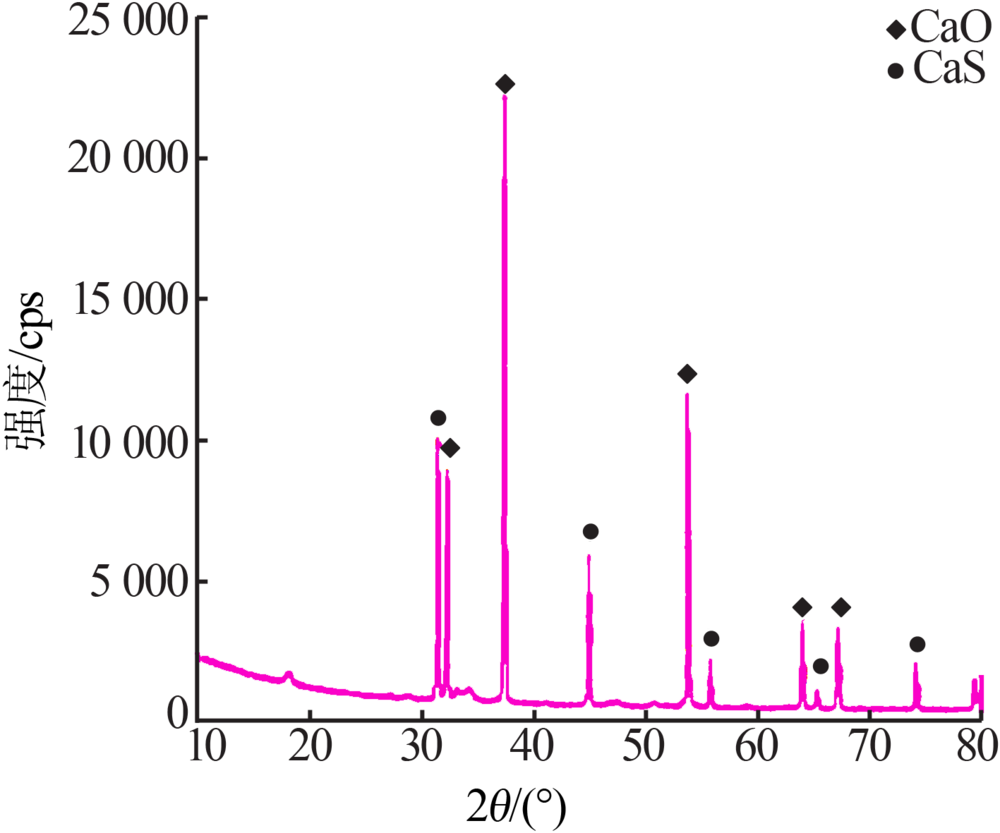
Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (7): 70-73.doi: 10.11962/1006-4990.2019-0474
• Environment·Health·Safety • Previous Articles Next Articles
Preparation of high purity calcium oxide combined with sulfuric acid from fluorine gypsum decomposed by sulfur
Zhang Yulu,Wang Ye( ),Yang Lin,Yang Xiushan,Wang Xinlong
),Yang Lin,Yang Xiushan,Wang Xinlong
- School of Chemical Engineering,Sichuan University,Chengdu 610065,China
-
Received:2020-01-11Online:2020-07-10Published:2020-07-13 -
Contact:Wang Ye E-mail:wangye@scu.edu.cn
CLC Number:
Cite this article
Zhang Yulu,Wang Ye,Yang Lin,Yang Xiushan,Wang Xinlong. Preparation of high purity calcium oxide combined with sulfuric acid from fluorine gypsum decomposed by sulfur[J]. Inorganic Chemicals Industry, 2020, 52(7): 70-73.
share this article
"
| 石膏种类 | 来源 | w(CaSO4)/% | 排放比例 (2016年)/%[ | 性能及用途 |
|---|---|---|---|---|
| 磷石膏 | 湿法磷酸副产品 | 59.7[ | 40.3 | 呈潮湿的细粉末状,自由水质量分数约为20%~30%。含有磷、氟、有机物等杂质,这些杂质对磷石膏及其建筑制品的性能影响很大,极大地限制了磷石膏的资源化利用[ |
| 脱硫石膏 | 火电厂烟气脱硫 | 68.8[ | 40.2 | 呈湿粉状,具有较高黏性。抗折强度与抗压强度明显高于天然石膏,可作为石膏板、水泥辅料、土壤改良剂、道路基层材料[ |
| 柠檬酸石膏 | 硫酸酸解柠檬酸钙产生的副产品 | 76.5[ | 2.94 | 外观白色(有时呈浅黄色),含水率为40%~60%,pH为5.0~6.2,拌杂有少量菌丝体;生产石膏粉、石膏板、水泥缓凝剂 |
| 盐石膏 | 盐场海水浓缩产生的副产品 | 71.5[ | 2.25 | 外观通常呈灰黄色,含有少量的无机盐类和大量泥沙,可作为水泥添加剂,建筑材料,石膏晶须[ |
| 钛石膏 | 硫酸法钛白生产副产品 | 51.2[ | 1.53 | 含水量高、黏度大、杂质含量高,应用于水泥缓凝剂、墙体材料、固定二氧化碳等方面[ |
| 芒硝石膏 | 由芒硝萃取硫酸钠或由钙芒硝生产芒硝的副产品 | 60.7[ | 1.60 | 芒硝石膏呈黄褐色或淡棕色,呈膏糊状,含水量一般在18%~28%,主要用途为水泥缓冲剂 |
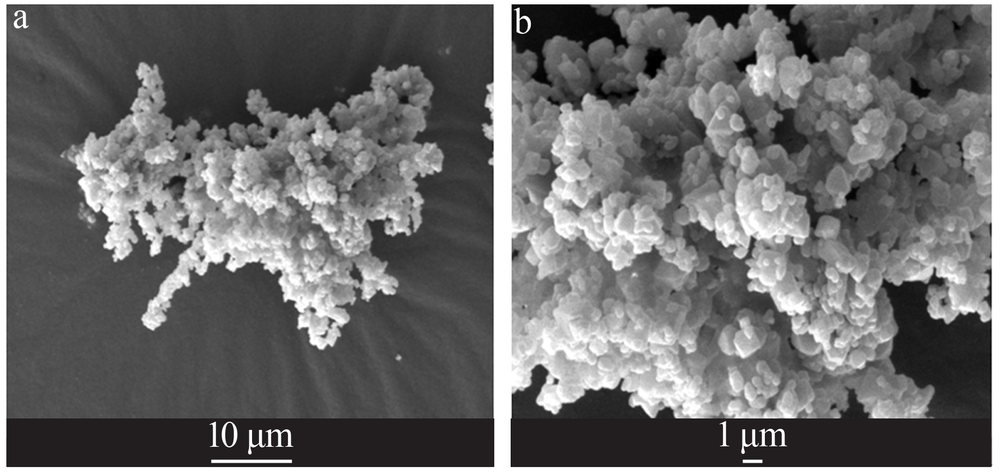
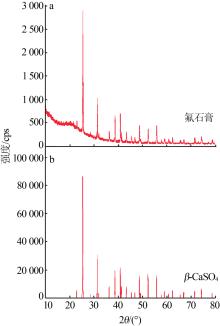
| 氟石膏 | 氢氟酸生产中,由硫酸与萤石反应产出的废渣 | 82.0[ | 0.74 | 呈灰白色干燥粉粒状、微晶状晶体,可作水泥缓凝剂、建筑粉刷石膏、新型墙体材料、制备硫酸钾 |
| [1] | 段鹏选, 张吉秀. 我国工业副产石膏行业发展概况[C]∥ “绿动百年,有你有我”环保产业发展高峰论坛论文集.邯郸:中国工业固废综合利用产业联盟, 2015. |
| [2] | 赵建华, 杨祥, 高士浩, 等. 石膏矿物学分析及除杂研究[J]. 硫酸工业, 2018(9):9-12,15. |
| [3] | 巴太斌, 徐亚中, 王利娜, 等. 磷石膏的改性及煅烧工艺研究综述[J]. 河南建材, 2018(1):22-24. |
| [4] | Pukalchik M A, Shadrin D, Katrutsa A, et al. Machine learning methods for estimation the indicators of phosphogypsum influence in soil[J]. Journal of Soils and Sediments, 2019(2):1-12. |
| [5] | 尹维新, 洪彩霞. 烟气脱硫石膏的基本性能分析及应用发展[J]. 太原科技, 2006(11):64-66,69. |
| [6] | Danielik V, Fellner P, Jurišová J, et al. Kinetics of the conversion reaction of gypsum with ammonium carbonate[J]. Chemical Papers, 2018,72:2631-2639. |
| [7] | 王传虎, 秦英月, 葛金龙, 等. 柠檬酸废渣石膏综合利用研究进展及前景展望[J]. 再生资源与循环经济, 2008(5):37-39. |
| [8] | 李志新, 徐开东, 牛季收, 等. 硅酸盐水泥提高盐石膏性能及其机理[J]. 硅酸盐通报, 2018,37(9):3017-3020. |
| [9] | 王胜春, 朱靖, 王红, 等. 我国海水制盐业废渣处理利用现状[J]. 河北理工学院学报, 2006,27(1):124-127. |
| [10] | 蒋美雪, 孙红娟, 彭同江, 等. 钛石膏硫酸浸取法提取铁质氧化物及石膏的物相变化[J]. 化工进展, 2019,38(4):2030-2036. |
| [11] | Zhang J, Yan Y, Hu Z. Preparation and characterization of foamed concrete with Ti-extracted residues and red gypsum[J]. Construction and Building Materials, 2018,171:109-119. |
| [12] | 董益宇. 芒硝石膏在水泥生产中的应用[J]. 四川建材, 2017,43(10):24-26. |
| [13] | 朱婧, 刘学敏, 邵丹娜, 等. 我国氟石膏资源化利用的现状及对策研究[J]. 资源开发与市场, 2013,29(4):410-413. |
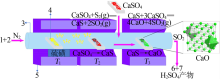
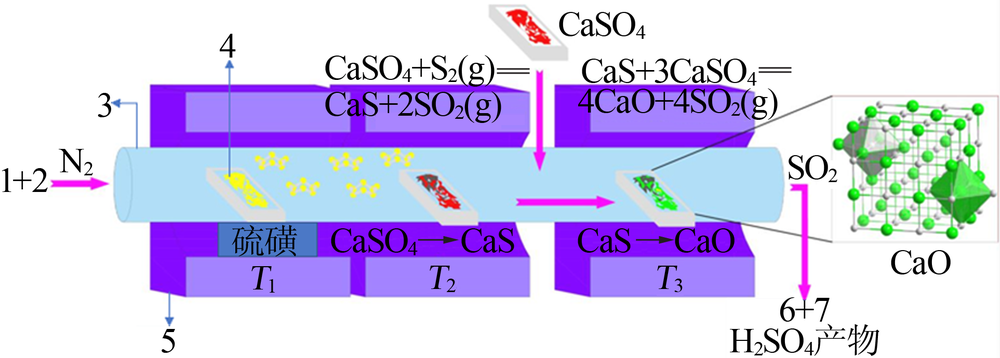
| [14] | 王辛龙, 张志业, 杨守明, 等. 硫磺分解磷石膏制硫酸技术进展及推广应用[J]. 硫酸工业, 2018(1):45-49,53. |
| [15] | 杨秀山, 刘荆风, 余家鑫, 等. 磷石膏制硫酸的研究进展[J]. 现代化工, 2010,30(9):8-12. |
| [16] | 郭文林, 刘智来, 杜晓荣, 等. 碘量法测定硫酸锰溶液中S2-含量[J]. 中国锰业, 2014,32(1):42-44. |
| [17] | 夏纯洁, 周觅, 沈琴芳, 等. 重量法测定石膏中三氧化硫的不确定度评定[J]. 化学通报, 2015,78(2):186-189. |
| [18] | 张静莉. 有效氧化钙测定方法的改进[J]. 化工设计通讯, 2018,44(8): 196, 203. |
| [1] | Feng Qing,Zeng Xianjun,Zhang Lijie,Li He,Meng Guangying,Sun Yanmin. Summary of transition metal manganese-based denitration catalyst for low temperature NH3-SCR [J]. Inorganic Chemicals Industry, 2021, 53(1): 7-13. |
| [2] | Lu Yuyuan,Xu Song,Su Tong,Yang Zhibin,Zhang Qiqin,Zhang Yang. Study on physical and chemical characteristics and adsorption performance of converter desulfurization slag [J]. Inorganic Chemicals Industry, 2021, 53(1): 87-90. |
| [3] | Jin Jihai,Liu Lizhi,Song Junhui,Jiao Zukai,Yan Jinlong,Zhen Tao,Zhang Duo. Study on hydrodeacidification performance of off-site pre-sulfidation catalysts [J]. Inorganic Chemicals Industry, 2020, 52(9): 100-104. |
| [4] | Wei Lan. Response surface methodology for optimizing production process parameters of polysulfur [J]. Inorganic Chemicals Industry, 2020, 52(9): 47-51. |
| [5] | Liu Yi,Ke Changmei,Yang Jintang,Zhang Jing,Chen Xintuo. Preparation of tetrabasic lead sulfate from desulfurized lead paste [J]. Inorganic Chemicals Industry, 2020, 52(9): 79-83. |
| [6] | Su Shaolong,Qu Xiaolong,Zhong Dule,Sun Yanmin,Nan Jun,Li Shipeng. Study on preparation and performance of desulfurizers in process of calcium injection desulfurization in furnace [J]. Inorganic Chemicals Industry, 2020, 52(7): 66-69. |
| [7] | Guo Ju,Jia Shuangzhu. Study of the method for the preparation of spherical-like and low sulfur FePO4 [J]. Inorganic Chemicals Industry, 2020, 52(5): 31-34. |
| [8] | Ke Haibo,Ke Changmei,Yu Jinzhao,Yang Jintang. Study on desulfurization process of waste lead paste by triethylamine process [J]. Inorganic Chemicals Industry, 2020, 52(4): 75-78. |
| [9] | Li Wenjun,Wang Jinyu,Li Hongling,Hong Chenglin,Dan Jianming,Qiao Xiuwen. Preparation of high concentration NaHSO3 solution by two-step method of desulfurization intermediate CaSO3 [J]. Inorganic Chemicals Industry, 2020, 52(3): 90-93. |
| [10] | Li Zhixin,Xu Kaidong,Zhou Hao,Wang Jina,Zhang Jianwu,Guo Gaowei. Study on composite admixtures modified salt gypsum [J]. Inorganic Chemicals Industry, 2020, 52(11): 75-78. |
| [11] | Zhou Jianwei,Cheng Baojun,Yu Baoying,Ma Pengfei,He Pingxian. Effect of heat-treated desulfurized gypsum on properties of super sulfated cement [J]. Inorganic Chemicals Industry, 2020, 52(11): 79-85. |
| [12] | Hu Min,Guo Jia,Wu Huadong,Zhang Linfeng. Photocatalytic oxidation of sodium sulfite in desulfurization wastewater by N-Zn/TiO2 [J]. Inorganic Chemicals Industry, 2020, 52(10): 151-156. |
| [13] | Liu Leilei,Xia Haijian,Feng Weiliang,Wang Tielin,Wang Cunwen,Wang Weiguo,Jiang Zhensheng. Determination and correlation of solubility of potassium fluosilicate in dilute sulfuric acid solution [J]. Inorganic Chemicals Industry, 2019, 51(8): 17-19. |
| [14] | Fang Yan,Dang Yagu,Zhang Shun. Economic analysis of sodium?鄄alkali desulfurization solution for improving double?鄄cycle alkali process [J]. Inorganic Chemicals Industry, 2019, 51(7): 58-60. |
| [15] | Sun Fengjuan,Liu Wanchao,Yan Kun,E Yishuai. Removal of chloride ion from desulfurization wastewater by ion-exchange resin [J]. Inorganic Chemicals Industry, 2019, 51(6): 45-48. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|