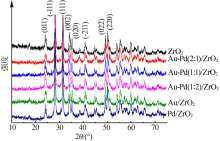
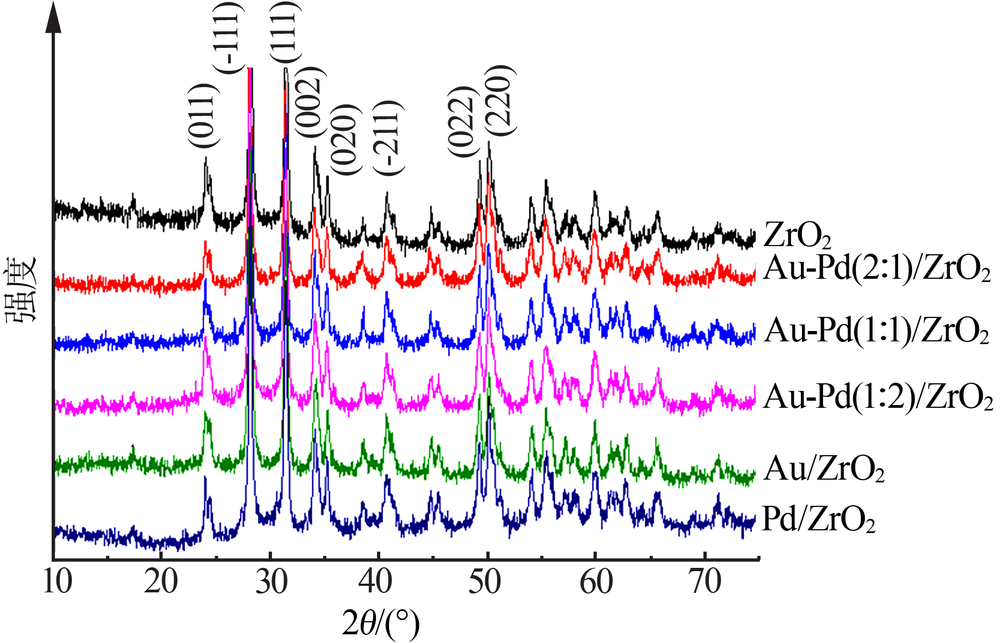
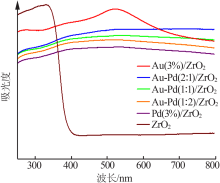
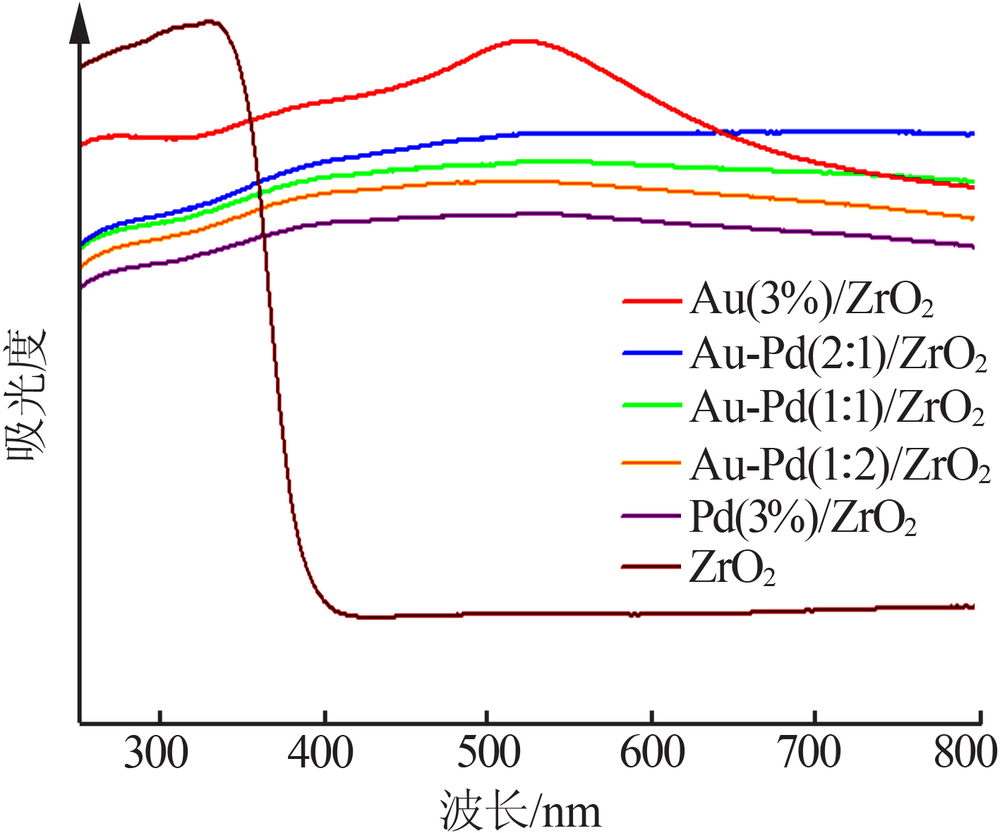
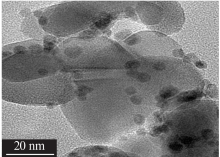
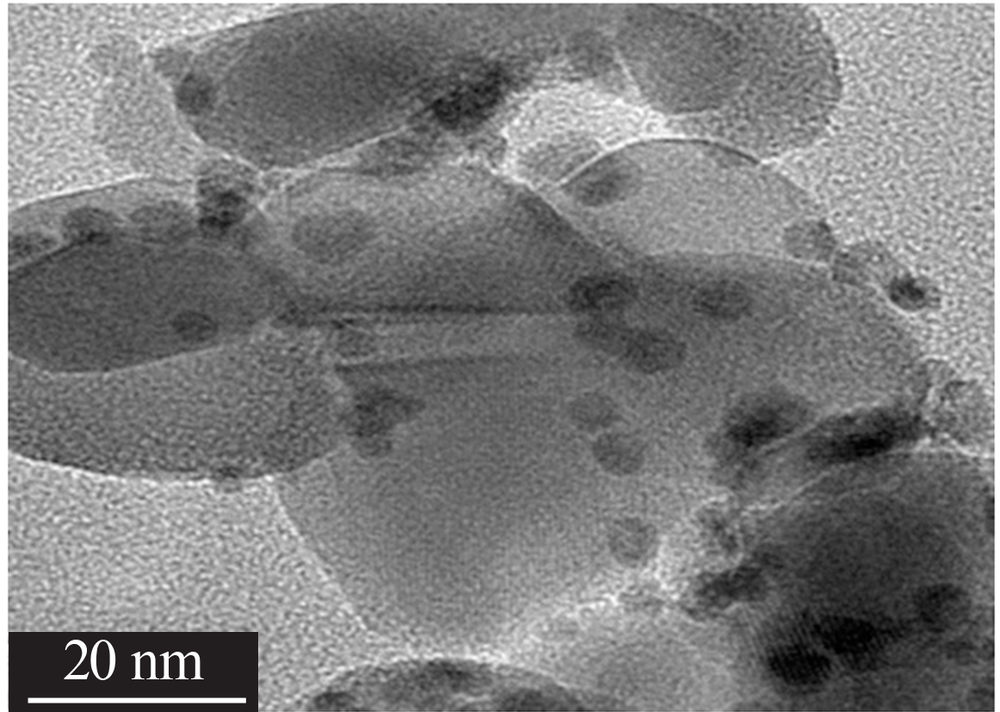
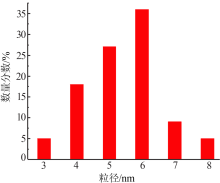
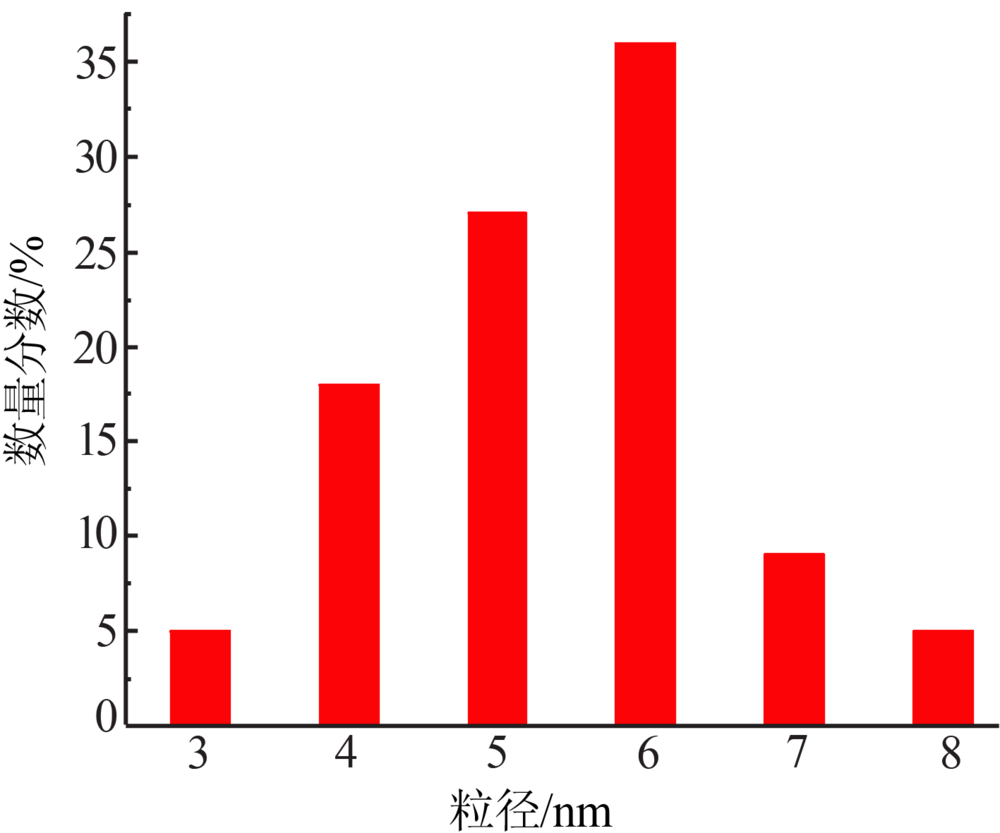
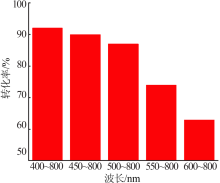
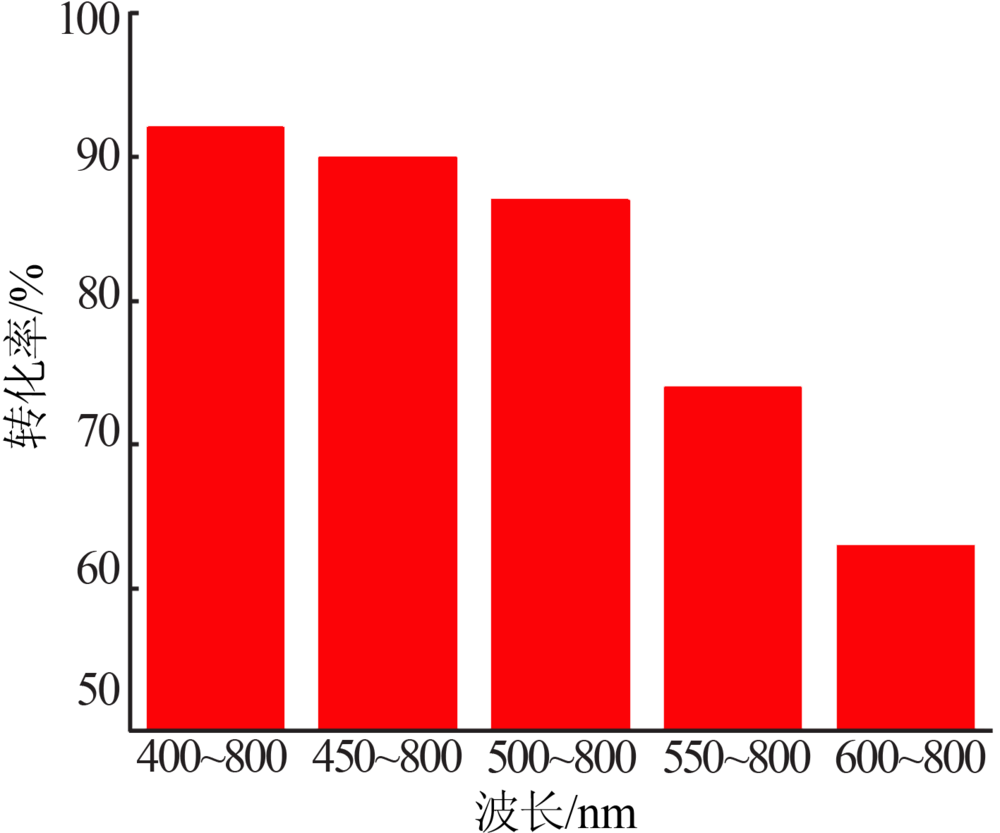
Inorganic Chemicals Industry ›› 2020, Vol. 52 ›› Issue (4): 100-103.doi: 10.11962/1006-4990.2019-0345
• Catalytic Materials • Previous Articles
Study on oxidation reacting conditions of benzyl alcohol catalyzed by Au-Pd/ZrO2 under visible light
Bai Hongliang1,Wang Junsheng2,Wang Yan3( )
)
- 1.Inner Mongolia Water Resources and Hydropower Survey and Design Institute,Hohhot 010010,China
2.Inner Mongolia Electric Power Research Institute
3.Inner Mongolia Vocational College of Chemical Engineeing
-
Received:2019-10-26Online:2020-04-10Published:2020-04-22 -
Contact:Wang Yan E-mail:654164408@qq.com
CLC Number:
Cite this article
Bai Hongliang,Wang Junsheng,Wang Yan. Study on oxidation reacting conditions of benzyl alcohol catalyzed by Au-Pd/ZrO2 under visible light[J]. Inorganic Chemicals Industry, 2020, 52(4): 100-103.
share this article
"
| 序号 | 催化剂 | 碱 | 产率/%(光照) | 产率/%(暗) |
|---|---|---|---|---|
| 1 | Au-Pd(2:1)/ZrO2 | Na2CO3 | 45.2 | 18.0 |
| 2 | Au-Pd(2:1)/ZrO2 | K2CO3 | 48.4 | 20.2 |
| 3 | Au-Pd(2:1)/ZrO2 | CsCO3 | 56.2 | 25.0 |
| 4 | Au-Pd(2:1)/ZrO2 | KOH | 90.2 | 35.0 |
| 5 | Au-Pd(2:1)/ZrO2 | NaOH | 88.5 | 31.0 |
| 6 | ZrO2 | KOH | 8.0 | 3.2 |
| 7 | Au/ZrO2 | KOH | 50.6 | 20.8 |
| 8 | Pd/ZrO2 | KOH | 20.2 | 8.2 |
| 9 | Au-Pd(1:1)/ZrO2 | KOH | 62.0 | 20.2 |
| 10 | Au-Pd(2:1)/ZrO2 | KOH | 90.2 | 35.0 |
| 11 | Au-Pd(1:2)/ZrO2 | KOH | 78.0 | 31.0 |
| [1] |
Huang G, Luo J, Deng C C , et al. Catalytic oxidation of toluene with molecular oxygen over mangmanese tetraphenlyporphyrin supported on chitosan[J]. Applied Catalysis A:General, 2008,338(1/2):83-86.
doi: 10.1016/j.apcata.2007.12.027 |
| [2] | Mitsudome T, Mikami Y, Funai H , et al. Oxidant-free alcohol dehy-drogenation using a reusable hydrotalcite-supported silver nano-particles catalyst[J]. Angew.Chem.Int.Ed., 2007,47(1):138-141. |
| [3] | Zhao G, Deng M, Jiang Y , et al. Microstructured Au/Ni-fiber cata-lyst;galvanic reaction preparation and catalytic performance for low-temperature gas-phase alcohol oxidation[J]. Journal of Cataly-sis, 2013,301:46-53. |
| [4] |
Fan J, Dai Y, Li Y , et al. Low-temperature,highly selective,gas-phase oxidation of benzyl alcohol over mesoporous K-Cu-TiO2 with stable copper(Ⅰ) oxidation state[J]. Journal of the American Che-mical Society, 2009,131(43):15568-15569.
doi: 10.1021/ja9032499 pmid: 19860474 |
| [5] | Bond G C, Sermon P A . Gold catalysts for olefin hydrogenation[J]. Gold Bull., 1973,6(4):102-105. |
| [6] | Haruta M, Kobayashi T, Sano H , et al. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 ℃[J]. Chem Lett., 1987,16(2):405-408. |
| [7] | Hutchings G J . Vapor phase hydorchlorination of acetylene:correla-tion of catalytic activity of supported metal chloride catalysts[J]. J.Catal., 1985,96(1):292-295. |
| [8] | Kawabata T, Shinozuka Y, Ohishi Y , et al. Nickel containing Mg-Al hydrotalcite-type anionic clay catalyst for the oxidation of alcohols with molecular oxygen[J]. J.Mol.Catal.A:Chem., 2005,236(1):206-215. |
| [9] |
Pina C D, Falletta E, Rossi M . Update on selective oxidation using gold[J]. Chem.Soc.Rev., 2012,41(1):350-369.
doi: 10.1039/c1cs15089h pmid: 21727977 |
| [10] |
McEwan L, Julius M, Roberts S , et al. A review of the use of gold ca-talysts in selective hydrogenation reactions Lynsey McEwana[J]. Gold Bull., 2010,43(4):298-306.
doi: 10.1007/BF03214999 |
| [11] | Yang X F, Wang A Q, Wang Y L , et al. Unusual selectivity of gold catalysts for hydrogenation of1,3-butadiene toward cis-2-butene:A joint experimental and theoretical investigation[J]. J.Phys.Chem.C, 2010,114(7):3131-3139. |
| [12] | Shimizu K, Sato R, Satsuma A . Direct C-C cross-coupling of secon-dary and primary alcohols catalyzed by a gamma-alumina-suppo-rted silver subnanocluster[J]. Angew.Chem.Int.Ed., 2009,48:3982-3986. |
| [13] |
Chen X, Zheng Z, Ke X , et al. Supported silver nanoparticles as photocatalysts under ultraviolet and visible light irradiation[J]. Green Chem., 2010,12(3):414-419.
doi: 10.1039/b921696k |
| [14] |
Fang W H, Chen J S, Zhang Q H , et al. Hydrotalcite-supported gold catalyst for the oxidant-free dehydrogenation of benzyl alcohol:studies on support and gold size effects[J]. Chemistry A European Journal, 2011,17(4):1247-1256.
doi: 10.1002/chem.201002469 pmid: 21243691 |
| [15] | Shao M W, Wang H, Zhang M L , et al. The mutual promotional ef-fect of Au-Pd bimetallic nanoparticles on silicon nanowires:A study of preparation and catalytic activity[J]. Appl.Phys.Lett., 2008,93:243110. |
| [16] |
Schunemann S, van Gastel M, Tüysüz H . A CsPbBr3/TiO2 compo-site for visible-light-driven photocatalytic benzyl alcohol oxidation[J]. ChemSusChem, 2018,11:2057-2061.
doi: 10.1002/cssc.201800679 pmid: 29790659 |
| [17] |
Song T, Park J E, Chung Y K . Rhodium-catalyzed synjournal of im-ines and esters from benzyl alcohols and nitroarenes:Chang in ca-talyst reactivity depending on the presence or absence of the pho-shine ligand[J]. The Journal of Organic Chemistry, 2018,83(7):4197-4203.
doi: 10.1021/acs.joc.8b00197 pmid: 29536727 |
| [18] | Wu J B, Shi R P, Qin Z F , et al. Selective oxidation of methanol to methyl formate over bimetallic Au-Pd nanoparticles supported on SiO2[J]. Journal of Fuel Chemistry and Technology, 2019,47(7):780-790. |
| [1] | Feng Fei,Li Shuwen,Wang Tielin,Wang Weiguo,Wang Cunwen. Synthesis and photocatalytic performance of sheet-like Bi/BiVO4 composite catalyst [J]. Inorganic Chemicals Industry, 2021, 53(1): 107-112. |
| [2] | Chen Lijia,Chen Haibin,Li Rongyong. Application progress of mechanical coating technique in preparation of photocatalytic materials [J]. Inorganic Chemicals Industry, 2020, 52(8): 1-5. |
| [3] | Zhang Shuli,Xu Xupeng,Chen Shurui,Liu Qianshu,Wang Benju. Photocatalytic activity of vanadium doped titanium dioxide [J]. Inorganic Chemicals Industry, 2020, 52(8): 93-97. |
| [4] | Zhou Jian,Tang Hongbo. Preparation and photocatalytic properties of ZnWO4/Ag3PO4 composite photocatalyst [J]. Inorganic Chemicals Industry, 2020, 52(6): 101-104. |
| [5] | Feng Yanxiang,Zhang Wangxi,Liang Baoyan. Study on synthesis of ZnO/g-C3N4 photocatalysts using solid phase reaction method and their photocatalytic property [J]. Inorganic Chemicals Industry, 2020, 52(10): 157-160. |
| [6] | Yin Nan,Liu Chanlu,Zhang Jin. Preparation and photocatalytic property of MoO3/g-C3N4 composite [J]. Inorganic Chemicals Industry, 2020, 52(10): 161-165. |
| [7] | Xiao Shanshan,Bi Fei,Zhao Li,Wang Liyan,Gai Guangqing. Research progress of cuprous oxide based composite photocatalyst [J]. Inorganic Chemicals Industry, 2020, 52(1): 22-25. |
| [8] | Zhang Shuai,Zhang Lisheng,Li Hui,Zhang Hanxin,Liang Jinglong. Progress in preparation process of TiO2 thin films [J]. Inorganic Chemicals Industry, 2019, 51(7): 15-18. |
| [9] | Li Shuwen,Zhou Yan,Wang Tielin. Study on preparation and photocatalysis reduction for CO2 of BiVO4/rGO composite [J]. Inorganic Chemicals Industry, 2019, 51(11): 82-87. |
| [10] | FAN Xue-Min, ZHANG Han-Chao, LI Guang-Hui, BAI Chun-Hua, XU Zhi-Yong, FAN Wen-Yang. Preparation and characterization of natural mineral loaded nano-sized TiO2 composites [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(7): 73-. |
| [11] | XU Xue-Tang, SU Hai-Yan, YU Liu-Hui, LIANG Yan-Yan, CHEN Kun, WANG Fan. Preparation and photocatalytic properties of Eu-doped BiVO4 micro-nano material [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(5): 61-. |
| [12] | CAI He-Shan, ZHUO Shao-Chun, HUANG Zhi, LI Hui-Shan, SUN Ming. Hydrothermal synthesis of Gd-doped potassium niobate and its photocatalytic activity to rhodamine degradation [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(5): 71-. |
| [13] | QUAN Yu-Lian, YANG Li-Jing, KANG Chun-Li. Effects of doping La3+ on photocatalytic activity of BiPO4 [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(5): 74-. |
| [14] | XU Xue-Tang, HUANG Bi-Fen, SU Hai-Feng, GOU Pan, SU Hai-Yan, WANG Fan. Hydrothermal synthesis and photocatalytic properties of Fe/Bi2WO6 [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(3): 72-. |
| [15] | XU Zhi-Yong, BAI Chun-Hua. Study on metal oxide doped kaolinite nano TiO2 photocatalytic composite [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(10): 77-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|