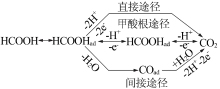
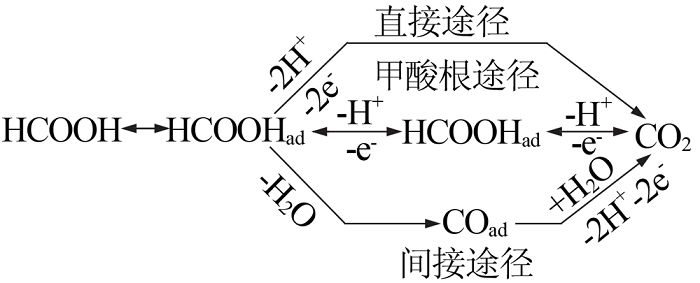
Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (5): 33-38.doi: 10.11962/1006-4990.2020-0306
• Reviews and Special Topics • Previous Articles Next Articles
Research progress on application of palladium-based catalyst in electrooxidation of formic acid
Chen Shaofeng1( ),Hou Lanfeng1,Liao Shijun2(
),Hou Lanfeng1,Liao Shijun2( )
)
- 1. Department of Chemical Engineering,Maoming Polytechnic,Maoming 525000,China
2. School of Chemistry and Engineering,South China University of Technology
-
Received:2020-08-29Online:2021-05-10Published:2021-05-12 -
Contact:Liao Shijun E-mail:cnshaofeng@qq.com;chsjliao@scut.edu.cn
CLC Number:
Cite this article
Chen Shaofeng,Hou Lanfeng,Liao Shijun. Research progress on application of palladium-based catalyst in electrooxidation of formic acid[J]. Inorganic Chemicals Industry, 2021, 53(5): 33-38.
share this article
| [1] |
Wang R, Liao S, Ji S. High performance Pd-based catalysts for oxi-dation of formic acid[J]. Journal of Power Sources, 2008,180(1):205-208.
doi: 10.1016/j.jpowsour.2008.02.027 |
| [2] |
Aslam N M, Masdar M S, Kamarudin S K, et al. Overview on direct formic acid fuel cells(DFAFCs) as an energy sources[J]. APCBEE Procedia, 2012,3:33-39.
doi: 10.1016/j.apcbee.2012.06.042 |
| [3] | 方向红, 张寅秋, 王琪, 等. Pd/AC催化剂催化分解甲酸反应条件的研究[J]. 无机盐工业, 2010,42(2):43-45. |
| [4] |
Brummer S B, Makrides A C. Adsorption and oxidation of formic acid on smooth platinum electrodes in perchloric acid solutions[J]. The Journal of Physical Chemistry, 1964,68(6):1448-1459.
doi: 10.1021/j100788a030 |
| [5] |
Ha S, Larsen R, Zhu Y, et al. Direct formic acid fuel cells with 600 mA/cm at 0.4 V and 22 ℃[J]. Fuel Cells, 2004,4(4):337-343.
doi: 10.1002/fuce.200400052 |
| [6] |
Rice C, Ha S, Masel R I, et al. Catalysts for direct formic acid fuel cells[J]. Journal of Power Sources, 2003,115(2):229-235.
doi: 10.1016/S0378-7753(03)00026-0 |
| [7] |
Cuesta A, Cabello G, Osawa M, et al. Mechanism of the electrocata-lytic oxidation of formic acid on metals[J]. ACS Catalysis, 2012,2(5):728-738.
doi: 10.1021/cs200661z |
| [8] |
Joo J, Uchida T, Cuesta A, et al. Importance of acid-base equilibrium in electrocatalytic oxidation of formic acid on platinum[J]. Journal of the American Chemical Society, 2013,135(27):9991-9994.
doi: 10.1021/ja403578s |
| [9] |
Chen Y X, Heinen M, Jusys Z, et al. Bridge-bonded formate:Active intermediate or spectator species in formic acid oxidation on a Pt film electrode?[J]. Langmuir, 2006,22(25):10399-10408.
pmid: 17129008 |
| [10] |
Chen Y X, Heinen M, Jusys Z, et al. Kinetics and mechanism of the electrooxidation of formic acid—Spectroelectrochemical studies in a flow cell[J]. Angewandte Chemie International Edition, 2006,45(6):981-985.
doi: 10.1002/(ISSN)1521-3773 |
| [11] | Hammer B, Nørskov J K. Theoretical surface science and cataly-sis—Calculations and concepts[J]. Advances in Catalysis, 2000,45:71-129. |
| [12] | 魏子栋. 质子交换膜燃料电池催化剂性能增强方法研究进展[J]. 化工进展, 2016,35(9):2629-2639. |
| [13] | Xu S. The electrooxidation of formic acid catalyzed by Pd-Ga nano-alloys[J]. Catalysis Science & Technology, 2019,9(5):1255-1259. |
| [14] |
Ulas B, Caglar A, Sahin O, et al. Composition dependent activity of PdAgNi alloy catalysts for formic acid electrooxidation[J]. Journal of Colloid and Interface Science, 2018,532:47-57.
doi: 10.1016/j.jcis.2018.07.120 |
| [15] | Zhang R, Yang M, Peng M, et al. Understanding the role of Pd:Cu ratio,surface and electronic structures in Pd-Cu alloy material applied in direct formic acid fuel cells[J]. Applied Surface Sci-ence, 2019,465:730-739. |
| [16] | Wang H, Li Y, Li C, et al. Hyperbranched PdRu nanospine assem-blies:An efficient electrocatalyst for formic acid oxidation[J]. Jo-urnal of Materials Chemistry A, 2018,36(6):17514-17518. |
| [17] | Kang X, Miao K, Guo Z, et al. PdRu alloy nanoparticles of solid so-lution in atomic scale:Size effects on electronic structure and ca-talytic activity towards electrooxidation of formic acid and meth-anol[J]. Journal of catalysis, 2018,364:18-191. |
| [18] | Zhang X, Fan H, Zheng J, et al. Pd-Zn nanocrystals for highly effi-cient formic acid oxidation[J]. Catalysis Science & Technology, 2018,18(8):4757-4765. |
| [19] |
Gong Y, Liu X, Gong Y, et al. Synjournal of defect-rich palladium-tin alloy nanochain networks for formic acid oxidation[J]. Journal of Colloid and Interface Science, 2018,530:189-195.
doi: 10.1016/j.jcis.2018.06.074 |
| [20] |
Wang S, Chang J, Xue H, et al. Catalytic stability study of a Pd-Ni2P/C catalyst for formic acid electrooxidation[J]. Chem.Electro.Chem., 2017,4(5):1243-1249.
doi: 10.1002/celc.201700051 |
| [21] |
Ma Y, Li T, Chen H, et al. A general strategy to the synjournal of car-bon-supported PdM(M=Co,Fe and Ni) nanodendrites as high-performance electrocatalysts for formic acid oxidation[J]. Journal of Energy Chemistry, 2017,26(6):1238-1244.
doi: 10.1016/j.jechem.2017.10.024 |
| [22] |
Zhang L Y, Liu Z. Graphene decorated with Pd4Ir nanocrystals:Ul-trasound-assisted synjournal,and application as a catalyst for oxidation of formic acid[J]. Journal of Colloid and Interface Science, 2017,505:783-788.
doi: S0021-9797(17)30749-X pmid: 28672257 |
| [23] | 何利华, 汪广进, 龚春丽, 等. 特殊形貌Pd纳米材料的控制合成及应用研究进展[J]. 材料导报, 2018(S2):44-49,68. |
| [24] | Bin D, Yang B, Ren F, et al. Facile synjournal of PdNi nanowire ne-tworks supported on reduced graphene oxide with enhanced cat-alytic performance for formic acid oxidation[J]. Journal of Materials Chemistry A, 2015,26(3):14001-14006. |
| [25] |
Zheng J N, Zhang M, Li F F, et al. Facile synjournal of Pd nanoch-ains with enhanced electrocatalytic performance for formic acid oxidation[J]. Electrochimica Acta, 2014,130:446-452.
doi: 10.1016/j.electacta.2014.03.054 |
| [26] |
Zhang L Y, Gong Y, Wu D, et al. Twisted palladium-copper nano-chains toward efficient electrocatalytic oxidation of formic acid[J]. Journal of Colloid and Interface Science, 2019,537:366-374.
doi: S0021-9797(18)31352-3 pmid: 30453230 |
| [27] |
Ortiz-Ortega E, Carrera-Cerritos R, Arjona N, et al. Pd Nanostruc-tures with high tolerance to CO poisoning in the formic acid elec-trooxidation reaction[J]. Procedia Chemistry, 2014,12:9-18.
doi: 10.1016/j.proche.2014.12.035 |
| [28] |
Xi Z, Erdosy D P, Mendoza-Garcia A, et al. Pd Nanoparticles cou-pled to WO2.72 nanorods for enhanced electrochemical oxidation of formic acid[J]. Nano Letters, 2017,17(4):2727-2731.
doi: 10.1021/acs.nanolett.7b00870 |
| [29] |
Yang F, Zhang Y, Liu P F, et al. Pd-Cu alloy with hierarchical ne-twork structure as enhanced electrocatalysts for formic acid oxidation[J]. International Journal of Hydrogen Energy, 2016,41(16):6773-6780.
doi: 10.1016/j.ijhydene.2016.02.145 |
| [30] | Zhang L Y, Gong Y, Wu D, et al. Palladium-cobalt nanodots ancho-red on graphene:In-situ synjournal,and application as an anode catalyst for direct formic acid fuel cells[J]. Applied Surface Sci-ence, 2019,469:305-311. |
| [31] |
Xu H, Zhang K, Yan B, et al. Ultra-uniform PdBi nanodots with high activity towards formic acid oxidation[J]. Journal of Power Sources, 2017,356:27-35.
doi: 10.1016/j.jpowsour.2017.04.070 |
| [32] |
Ma T, Li C, Liu T, et al. Size-controllable synjournal of dendritic Pd nanocrystals as improved electrocatalysts for formic acid fuel cells′ application[J]. Journal of Saudi Chemical Society, 2018,22(7):846-854.
doi: 10.1016/j.jscs.2018.01.007 |
| [33] |
Wang H H, Zhang J F, Chen Z L, et al. Size-controllable synjournal and high-performance formic acid oxidation of polycrystalline Pd nanoparticles[J]. Rare Metals, 2019,38(2):115-121.
doi: 10.1007/s12598-017-0947-0 |
| [34] |
Liu X, Li Z, Wang K, et al. Facile synjournal of Pd nanocubes with assistant of iodide and investigation of their electrocatalytic perf-ormances towards formic acid oxidation[J]. Nanomaterials, 2019,9(3):375.
doi: 10.3390/nano9030375 |
| [35] |
Yazdan-Abad M Z, Noroozifar M, Alfi N. Investigation on the elec-trocatalytic activity and stability of three-dimensional and two-di-mensional palladium nanostructures for ethanol and formic acid oxidation[J]. Journal of Colloid and Interface Science, 2018,532:485-490.
doi: S0021-9797(18)30909-3 pmid: 30103131 |
| [36] |
Qiu X, Zhang H, Wu P, et al. One-pot synjournal of freestanding po-rous palladium nanosheets as highly efficient electrocatalysts for formic acid oxidation[J]. Advanced Functional Materials, 2017,27(1).Doi: 10.1002/adfm.201603852.
doi: 10.1002/adfm.201603852 |
| [37] |
Cazares-ávila E, Ruiz-Ruiz E J, Hernández-Ramírez A, et al. Ef-fect of OMC and MWNTC support on mass activity of PdCo cata-lyst for formic acid electro-oxidation[J]. International Journal of Hydrogen Energy, 2017,42(51):30349-30358.
doi: 10.1016/j.ijhydene.2017.08.156 |
| [38] |
Mazurkiewicz-Pawlicka M, Malolepszy A, Mikolajczuk-Zychora A, et al. A simple method for enhancing the catalytic activity of Pd deposited on carbon nanotubes used in direct formic acid fuel cells[J]. Applied Surface Science, 2019,476:806-814.
doi: 10.1016/j.apsusc.2019.01.114 pmid: WOS:000459458600097 |
| [39] |
Lu H T, Yang Z J, Yang X, et al. CeO2 Nanotubes supported Pd elec-trocatalysts for formic acid oxidation[J]. Electrocatalysis, 2015,6(3):255-262.
doi: 10.1007/s12678-014-0239-5 |
| [40] | Ali H, Kanodarwala F K, Majeed I, et al. La2O3 Promoted Pd/rGO electro-catalysts for formic acid oxidation[J]. ACS Applied Materi-als & Interfaces, 2016,8(47):32581-32590. |
| [41] |
Kankla P, Limtrakul J, Green M L H, et al. Electrooxidation of for-mic acid enhanced by surfactant-free palladium nanocubes on sur-face modified graphene catalyst[J]. Applied Surface Science, 2019,471:176-184.
doi: 10.1016/j.apsusc.2018.12.001 |
| [42] | 吕美英, 李文鹏, 刘慧玲, 等. 利用石墨烯-炭黑组成的二元碳载体增强甲酸在钯催化剂上电化学氧化的活性[J]. 催化学报, 2017,38(5):939-947. |
| [43] | Ko Y J, Kim J Y, Lee K S, et al. Palladium nanoparticles from sur-factant/fast-reduction combination one-pot synjournal for the liquid fuel cell applications[J]. International Journal of Hydrogen Ener-gy, 2018,43(41):19029-19037. |
| [44] |
Gong Y, He N, Qin C, et al. Nitrogen-doped carbon-modified tita-nium oxides supported Pd catalyst for the electrooxidation of for-mic acid[J]. Journal of Solid State Electrochemistry, 2018,22(8):2623-2628.
doi: 10.1007/s10008-018-3948-5 |
| [45] | Xu H, Yan B, Zhang K, et al. N-doped graphene-supported binary PdBi networks for formic acid oxidation[J]. Applied Surface Sci-ence, 2017,416:191-199. |
| [1] | LUO Chengling, FAN Xiaofan. Research progress of microstructure-regulated catalysts for urea oxidation reactions [J]. Inorganic Chemicals Industry, 2025, 57(2): 26-35. |
| [2] | LIU Qingcui, LI Yunqing, PANG Ruiqi, TIAN Yaping, CHEN Yiying, LI Fang, LI Qiming. Preparation of Zn/Co-ZIF derived porous carbon supported Pd as catalyst and its application to formic acid dehydrogenation [J]. Inorganic Chemicals Industry, 2024, 56(6): 147-152. |
| [3] | HAN Xiudong. Research progress on preparation method of platinum based ordered nano catalysts [J]. Inorganic Chemicals Industry, 2022, 54(5): 47-53. |
| [4] | ZHENG Yangzi,JIN Mingshang. Strategy to improve catalytic performance of Pt-based core-shell catalysts for fuel cells [J]. Inorganic Chemicals Industry, 2022, 54(11): 1-7. |
| [5] | YOU Hongxin,WANG Qiang,PENG Lian. Performance and characterization of Ni-Fe/Ce0.9Gd0.1O1.95 composite anode for cathode-supported single cell [J]. Inorganic Chemicals Industry, 2022, 54(1): 34-38. |
| [6] | Du Miao,Ma Zhiyuan,Ji Changjian,Wang Lei. Research progress of nitrogen-doped carbon nanomaterials for ORR catalyst [J]. Inorganic Chemicals Industry, 2021, 53(6): 72-78. |
| [7] | Zhang Jie,Zhao Mengjie,Cui Yingqi,Li Chenggang,Gao Jinhai. Research progress in effect of doping on properties of CeO2-based electrolyte materials [J]. Inorganic Chemicals Industry, 2020, 52(5): 1-5. |
| [8] | Bian Chengli,Shi Huangang,Bao Wenyun,Zhang Dongping. Heat treatment of FGD gypsum and preparation of molds for slip casting [J]. Inorganic Chemicals Industry, 2020, 52(2): 54-57. |
| [9] | GAO Hui-Min, LIANG Xue-Song, MEI Yi, YANG Ya-Bin, ZHU Yan-Jin, YANG Yun-Song. Research on separation technology of formic acid produced from by-product sodium formate of phosphorus tail gas [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(1): 42-. |
| [10] | Fang Xianghong;Zhang Yinqiu;Wang Qi;Cui Peng. Study on reaction condition of catalytic decomposition of HCOOH with Pd/AC catalyst [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(2): 0-0. |
| [11] | Zhang Xiang;Sun kuibin;Zhou Junbo. Progress in hydrogen production technology from hydrolysis of sodium borohydride [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(1): 0-0. |
| [12] | Liu Xianglai. Discussion on problems about palladium catalyst used in production of hydrogen peroxide [J]. INORGANICCHEMICALSINDUSTRY, 2009, 0(4): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||