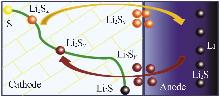
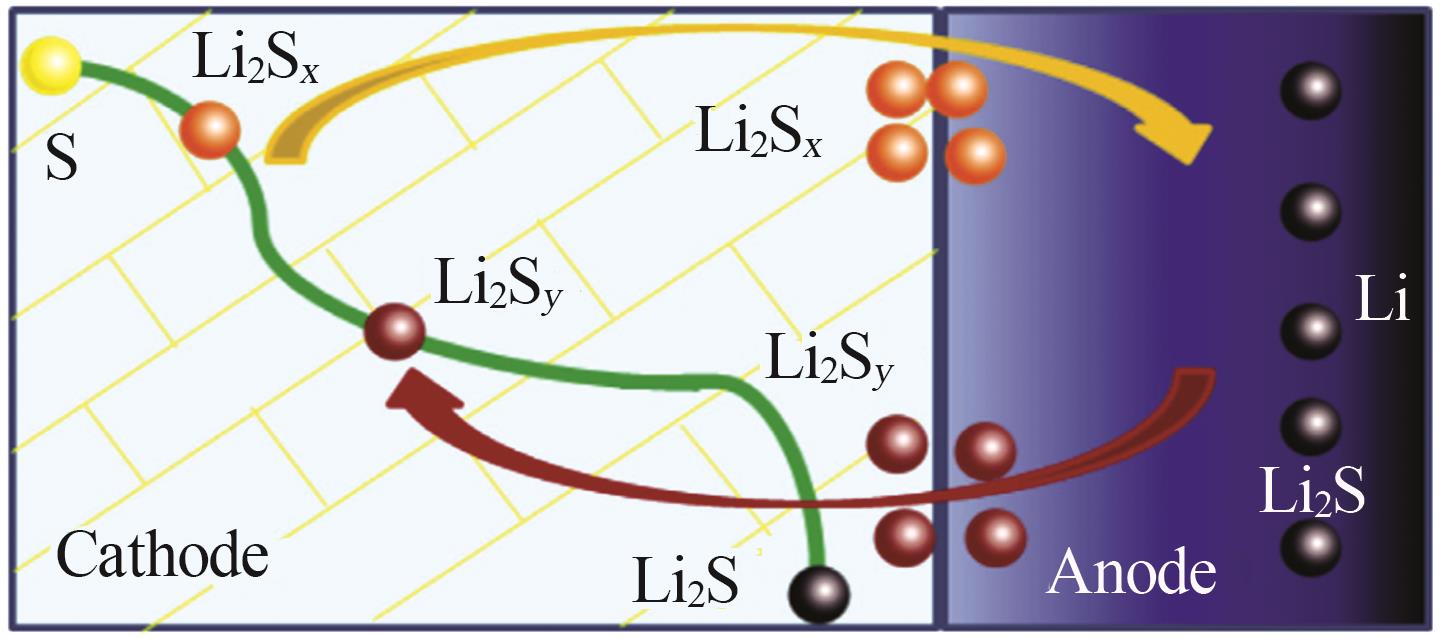
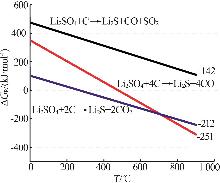
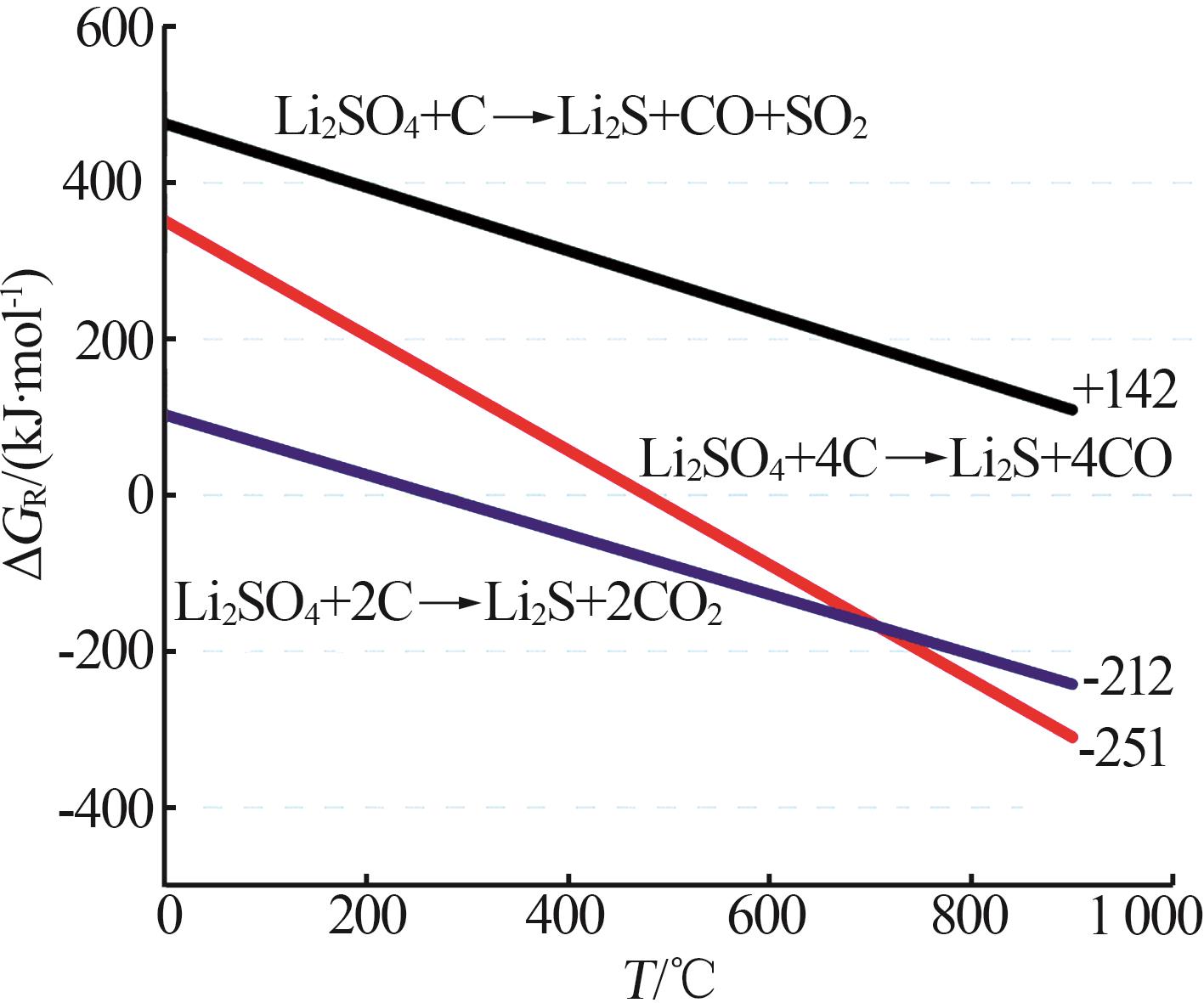
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (8): 12-20.doi: 10.19964/j.issn.1006-4990.2024-0517
• Reviews and Special Topics • Previous Articles Next Articles
Research progress on preparation of lithium sulphide key material for all-solid-state batteries
SUN Jiale1( ), TIAN Huan1,2, LEI Zhen1, YANG Liu1, ZHONG Zhaozi1, CHEN Ge1,2(
), TIAN Huan1,2, LEI Zhen1, YANG Liu1, ZHONG Zhaozi1, CHEN Ge1,2( )
)
- 1.Tianqi Lithium(shehong)Co. ,Ltd. ,Shehong 629200,China
2.Sichuan Key Laboratory of Lithium Resources and Materials,Shehong 629200,China
-
Received:2024-09-29Online:2025-08-10Published:2025-01-02 -
Contact:CHEN Ge E-mail:sunjl@tianqilithium.com;chenge@tianqilithium.com
CLC Number:
Cite this article
SUN Jiale, TIAN Huan, LEI Zhen, YANG Liu, ZHONG Zhaozi, CHEN Ge. Research progress on preparation of lithium sulphide key material for all-solid-state batteries[J]. Inorganic Chemicals Industry, 2025, 57(8): 12-20.
share this article
Table 1
Comparison of lithium sulfide laboratory synthesis methods"
| 合成方法 | 原料 | 优点 | 缺点 |
|---|---|---|---|
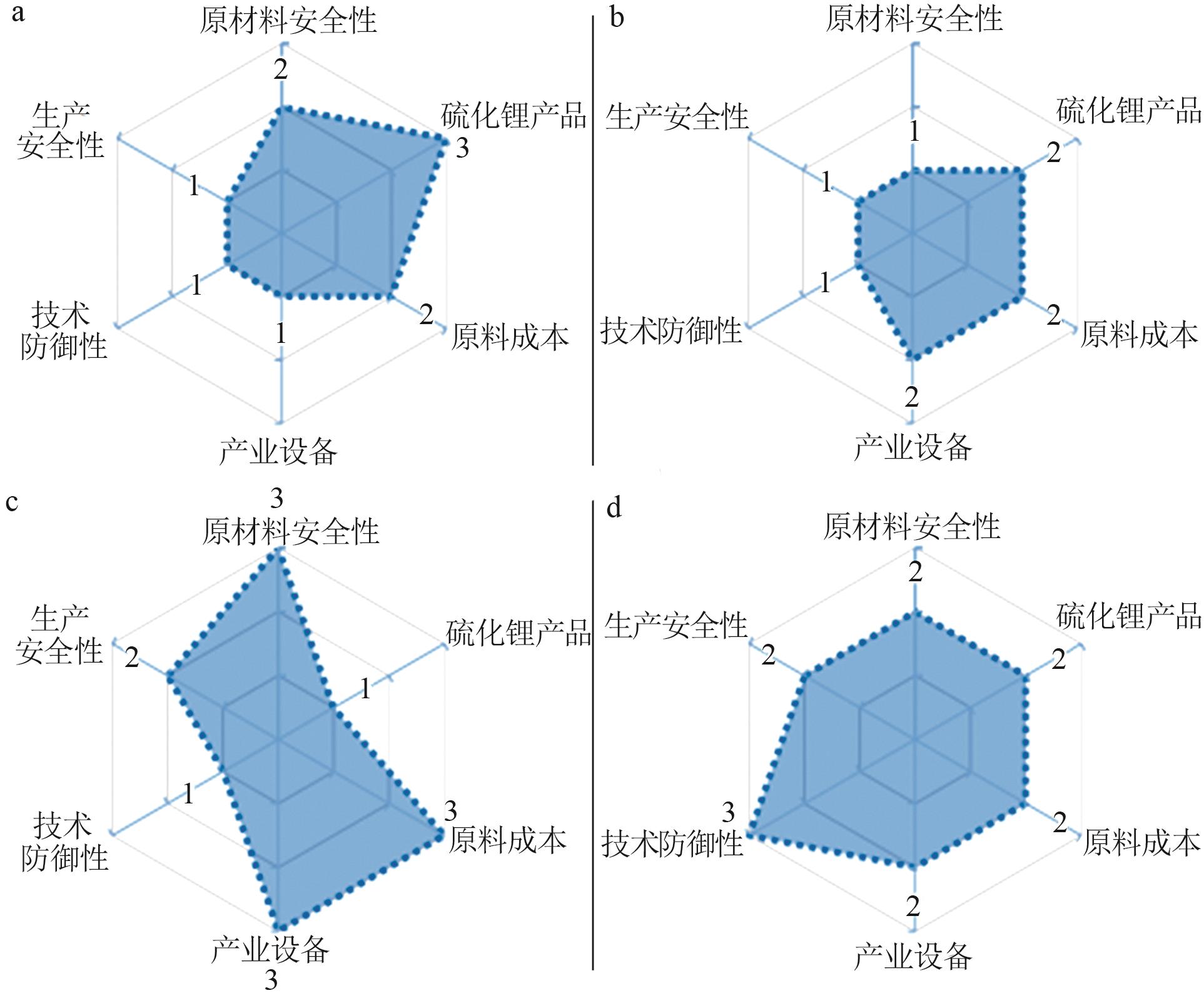
| 球磨法 | 金属锂、氨基锂、亚氨基锂、氮化锂硫粉 | 工艺简单,环境友好,无废液产生 | 原料成本高,反应时间长,转化率较低,所得产品存在杂质,提纯复杂,产业化设备不易选型 |
| 液相法 | 金属锂、氢氧化锂、萘化锂、丁基锂硫粉、硫化氢、乙醇、己烷、甲苯、乙醚、四氢呋喃、N-甲基吡咯烷酮 | 液相反应充分完全,不易残留杂质,产品提纯容易;不需要高温处理,能耗较小;工艺简单,工况较易控制 | 有机溶剂易燃、易爆、易挥发,环境污染严重,不易回收;工况危险性高,较难控制 |
高温/ 高压法 | 金属锂、氢氧化锂、碳酸锂硫蒸汽、硫化氢 | 工艺流程简单,且有效利用高温高压密闭反应的优势,避免有害溶剂泄漏,大大缩短制备流程 | 高温、高压,工况不易控制,设备选型要求高,增加了反应过程及后处理的风险 |
碳热 还原法 | 硫酸锂、碳材料、乙醇 | 反应更易控制,解决了因硫化锂遇水、氧敏感而导致的生产和储运困难的问题;提高产品收率和性能;改善传统硫化锂/碳复合材料制备工序复杂的现状;提高活性材料在锂硫电池正极的分散性;提升锂硫电池的电化学性能 | 工艺技术尚需优化完善,产品质量不稳定,复合材料形貌可控性较差 |
Table 2
Comparison of domestic and foreign mainstream lithium sulfide production technology"
| 制备方法 | 代表专利 | 优缺点 |
|---|---|---|
金属锂和 硫法 | CN,112607712A[ CN,112777571A[ | 优点:产品品质高,环境友好、无废液产生 缺点:反应温度较高,设备使用寿命短,反应不可控,风险极高 |
| 硫化氢和氢氧化锂法 | EP,3235787B1[ JP,2016094341A[ JP,1997278423A[ CN,117396427A[ JP,2015137183A[ | 优点:原料成本低 缺点:使用剧毒硫化氢气体,设备选型要求高 |
碳热 还原法 | CN,114275742A[ | 优点:原料获取方便,生产成本低工艺操作简单 缺点:产品中碳含量高,产品品质含量较低 |
浆态 还原法 | CN,117069067A[ | 优点:反应温度温和,工艺操作简单,安全性高,生产成本低 缺点:有废液和废气产生 |
| [1] | NEUMANN J, PETRANIKOVA M, MEEUS M,et al.Recycling of lithium-ion batteries:Current state of the art,circular economy,and next generation recycling[J].Advanced Energy Materials,2022,12(17):2102917. |
| [2] | BRUCE P G, FREUNBERGER S A, HARDWICK L J,et al.Li-O2 and Li-S batteries with high energy storage[J].Nature Materials,2011,11(1):19-29. |
| [3] | WU Yujing, XU Jing, LU Pushun,et al.Thermal stability of sulfide solid electrolyte with lithium metal[J].Advanced Energy Materials,2023,13(36):2301336. |
| [4] | YANG Shijie, HU Jiangkui, JIANG Fengni,et al.Oxygen-induced thermal runaway mechanisms of Ah-level solid-state lithium metal pouch cells[J].eTransportation,2023,18:100279. |
| [5] | WANG Yue, WU Yujing, WANG Zhixuan,et al.Doping strategy and mechanism for oxide and sulfide solid electrolytes with high ionic conductivity[J].Journal of Materials Chemistry A,2022,10(9):4517-4532. |
| [6] | LIANG Xin, WANG Lulu, WU Xiaolong,et al.Solid-state electrolytes for solid-state lithium-sulfur batteries:Comparisons,advances and prospects[J].Journal of Energy Chemistry,2022,73:370- 386. |
| [7] | ZHU Hongzheng, LIU Jian.Emerging applications of spark plasma sintering in all solid-state lithium-ion batteries and beyond[J].Journal of Power Sources,2018,391:10-25. |
| [8] | KIM M S, GWON H J, JO N J.Oligo(EDOT)/PVdF blend electrolyte for all solid polymer battery[J].Applied Chemistry for Engineering,2022,33(3):289-295. |
| [9] | LI Zhuo, FU Jialong, ZHOU Xiaoyan,et al.Ionic conduction in polymer-based solid electrolytes[J].Advanced Science,2023,10(10):2201718. |
| [10] | MURALI A, SAKAR M, PRIYA S,et al.Insights into the emerging alternative polymer-based electrolytes for all solid-state lithi- |
| um-ion batteries:A review[J].Materials Letters,2022,313:131764. | |
| [11] | ZHAO Xiaoxue, WANG Chao, LIU Hong,et al.A review of polymer-based solid-state electrolytes for lithium-metal batteries:Structure,kinetic,interface stability,and application[J].Batteries & Supercaps,2023,6(4):e202200502. |
| [12] | JIANG Pengfeng, DU Guangyuan, CAO Jiaqi,et al.Solid-state Li ion batteries with oxide solid electrolytes:Progress and perspective[J].Energy Technology,2023,11(3):2201288. |
| [13] | RAJAGOPAL R, SUBRAMANIAN Y, JUNG Y J,et al.Preparation of metal-oxide-doped Li7P2S8Br0.25I0.75 solid electrolytes for all-solid-state lithium batteries[J].ACS Applied Materials & Interfaces,2023,15(17):21016-21026. |
| [14] | LIU Mian, GUAN Xiang, LIU Hongmei,et al.Composite solid electrolytes containing single-ion lithium polymer grafted garnet for dendrite-free,long-life all-solid-state lithium metal batteries[J].Chemical Engineering Journal,2022,445:136436. |
| [15] | ZHANG Yue, ZHAI Wenbo, HU Xiangchen,et al.Application of Auger electron spectroscopy in lithium-ion conducting oxide solid electrolytes[J].Nano Research,2023,16(3):4039-4048. |
| [16] | WU Jinghua, LIU Sufu, HAN Fudong,et al.Lithium/sulfide all-solid-state batteries using sulfide electrolytes[J].Advanced Materials,2021,33(6):2000751. |
| [17] | ATHANASIOU C E, LIU Xing, JIN M Y,et al.Rate-dependent deformation of amorphous sulfide glass electrolytes for solid-state batteries[J].Cell Reports Physical Science,2022,3(4):100845. |
| [18] | CAO Yi, ZUO Pengjian, LOU Shuaifeng,et al.A quasi-solid-state Li-S battery with high energy density,superior stability and safety[J].Journal of Materials Chemistry A,2019,7(11):6533-6542. |
| [19] | JI Xiulei, LEE K T, NAZAR L F.A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries[J].Nature Materials,2009,8(6):500-506. |
| [20] | HIESGEN R, SÖRGEL S, COSTA R,et al.AFM as an analysis tool for high-capacity sulfur cathodes for Li-S batteries[J].Beilstein Journal of Nanotechnology,2013,4:611-624. |
| [21] | WIHEEB A D, SHAMSUDIN I K, AHMAD M A,et al.Present technologies for hydrogen sulfide removal from gaseous mixtures[J].Reviews in Chemical Engineering,2013,29(6):449- 470. |
| [22] | CHENG Xinbing, HUANG Jiaqi, PENG Hongjie,et al.Polysulfide shuttle control:Towards a lithium-sulfur battery with superior capacity performance up to 1000 cycles by matching the sulfur/electrolyte loading[J].Journal of Power Sources,2014,253:263-268. |
| [23] | 李良彬,刘小康,廖萃,等.一种利用金属锂制备硫化锂的方法:CN,112607712A[P].2021-04-06. |
| [24] | 梁初,胡梦茹,王凯,等.一种硫化锂的绿色高效制备方法:CN,112125322A[P].2020-12-25. |
| [25] | 韩金龙,陆子恒,杨春雷.一种硫化锂的制备方法:CN,114394608A[P].2022-04-26. |
| [26] | 何志达,朱刘,高远,等.一种高纯硫化锂的制备方法及装置:CN,105016310A[P].2015-11-04. |
| [27] | 周复,杨柳,陈格,等.硫化锂的制备方法:CN,112678781A [P]. 2021-04-20. |
| [28] | LI Xuemin, WOLDEN C A, BAN Chunmei,et al.Facile synthesis of lithium sulfide nanocrystals for use in advanced rechargeable batteries[J].ACS Applied Materials & Interfaces,2015,7(51):28444-28451. |
| [29] | FANG Liran, ZHANG Qiaran, HAN Aiguo,et al.Green synthesis of the battery material lithium sulfide via metathetic reactions[J].Chemical Communications,2022,58(36):5498-5501. |
| [30] | 山本一富,池田信彦.硫化リチウムの製造方法:JP,1997278423A[P].1997-10-28. |
| KAZUTOMI Yamamoto, NOBUHIKO Ikeda.Production of lithium sulfide:JP,1997278423A[P].1997-10-28. | |
| [31] | 山本一富,池田信彦,植松 敏勝.硫化リチウムの製造方法:JP,1997110404A[P].1997-04-28. |
| KAZUTOMI Yamamoto, NOBUHIKO Ikeda, TOSHIKATSU Uematsu.Production of lithium sulfide:JP,1997110404A[P].1997-04-28. | |
| [32] | 罗发洪,朱辰.一种硫化锂的制备方法:CN,108400327A[P]. 2018-08-14. |
| [33] | KOHL M, BRÜCKNER J, BAUER I,et al.Synthesis of highly electrochemically active Li2S nanoparticles for lithium-sulfur-batteries[J].Journal of Materials Chemistry A,2015,3(31):16307-16312. |
| [34] | YE Fangmin,NOH H, LEE Jinhong,et al.Li2S/carbon nanocomposite strips from a low-temperature conversion of Li2SO4 as high-performance lithium-sulfur cathodes[J].Journal of Materials Che- mistry A,2018,6(15):6617-6624. |
| [35] | ZHANG Yong, XIE Mengxiang, ZHANG Wu,et al.Synthesis and purification of SiS2 and Li2S for Li9.54Si1.74P1.44S11.7Cl0.3 solid electrolyte in lithium-ion batteries[J].Materials Letters,2020,266:127508. |
| [36] | 杨永安,张欣.一种电池级硫化锂的制备方法:CN,114477099A[P].2022-05-13. |
| [37] | 彭文修,亓亮,吕延鹏,等.一种合成电池级硫化锂的方法:CN,112777571A[P].2021-05-11. |
| [38] | SENGA M, IWAHARA M.Device for producing lithium sulfide,and method for producing lithium sulfide:US,10239027[P]. 2019-03-26. |
| [39] | 柳和明,千賀実,油谷亮.硫化リチウムの製造方法:JP,2016094341A[P].2016-05-26. |
| Yanagi Kazuaki, Senga Minoru, Aburaya Ryo.Producing method of lithium sulfide:JP,2016094341A[P].2016-05-26. | |
| [40] | 后藤裕辉,山本一富.硫化锂制造装置以及硫化锂的制造方法:CN,117396427A[P].2024-01-12. |
| [41] | 諫山篤,加藤秀利.硫化リチウムの製造方法:JP,2015137183A [P].2015-07-30. |
| Isayama Atsushi, Kato Hidetoshi.Method of producing lithium sulfide:JP,2015137183A [P].2015-07-30. | |
| [42] | 김은주.유동층 반응기를 이용한 황화리튬의 건식 연속 생산 시스템:KR,1020240058647A[P].2024-05-03. |
| KIM Eunjoo.A fluidized bed reactor system capable of continuous dry production of lithium sulfide used in solid electrolytes:KR,1020240058647A[P].2024-05-03. | |
| [43] | 刘芳洋,杜岳勇,张宗良.一种可实现连续化生产的硫化锂的制备方法:CN,114275742A[P].2022-04-05. |
| [44] | 徐川,陈格,田欢,等.EV级高纯硫化锂及其制备方法:CN,117069067A[P].2023-11-17. |
| [1] | LI Junxi, XIE Zhipeng, LIU Yunfeng, MA Long, CHEN Jiale, ZHANG Da, YANG Bin, LIANG Feng. Research progress of preparation of metal borides and their military applications [J]. Inorganic Chemicals Industry, 2024, 56(12): 13-28. |
| [2] | ZHANG Liyuan,SHEN Ruqian,YANG Jinju,LI Yan,SHUI Yi,SU Min. Research progress on lithium ion sieves [J]. Inorganic Chemicals Industry, 2022, 54(5): 28-37. |
| [3] | DONG Jia-Li, WANG Lu-Xun, LI Jian, YANG Li-Na, XU Long, SUN Yu-Meng. Research progress of preparation of bimodal mesoporous silica [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(6): 17-. |
| [4] | HAN Dong-Zhan, YIN Zhong-Lin, WANG Jian-Li- . Research progress in preparation and application of high purity alumina [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(9): 1-. |
| [5] | XU Jin-Yao, MING Da-Zeng, LI Zhi-Xiang, HE Hong-Liang. Preparation methods of magnesium fluoride [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(9): 8-. |
| [6] | PENG Ai-Guo, HE Zhou-Chu, XIAO Wei, DING Xiong-Lei, ZHUANG Xin-Juan. Research progress of chemical manganese dioxide [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(3): 8-. |
| [7] | Li Suying;Qian Haiyan. Preparation methods and application actuality of silica white [J]. INORGANICCHEMICALSINDUSTRY, 2008, 0(1): 0-0. |
| [8] | Mao Jinlong;Guo Shenghui;Peng Jinhui;Zhang Libo;Zhang Shimin;Chen Guo. Phase stabilization and preparation methods of zirconia [J]. INORGANICCHEMICALSINDUSTRY, 2008, 0(1): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||