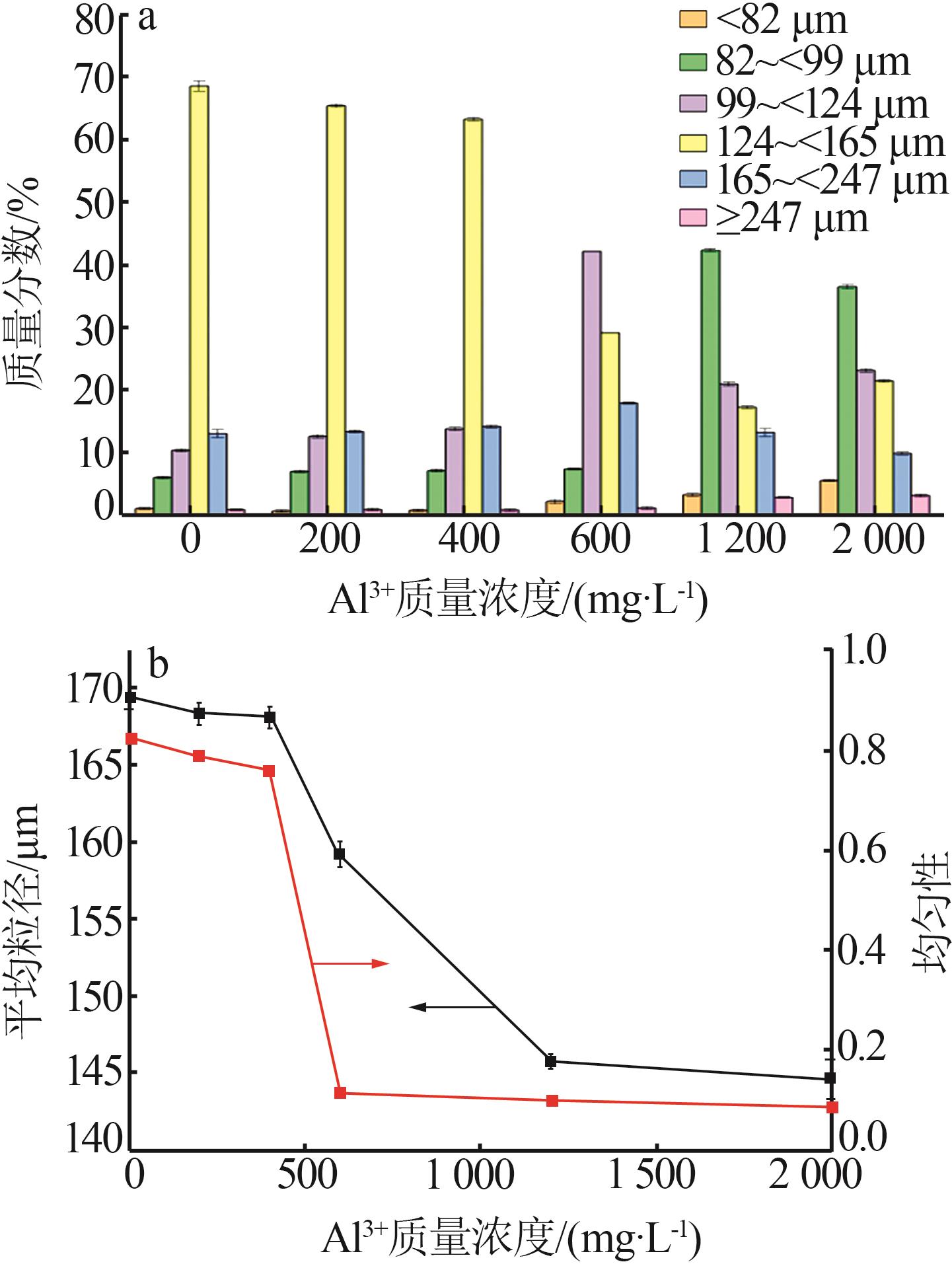
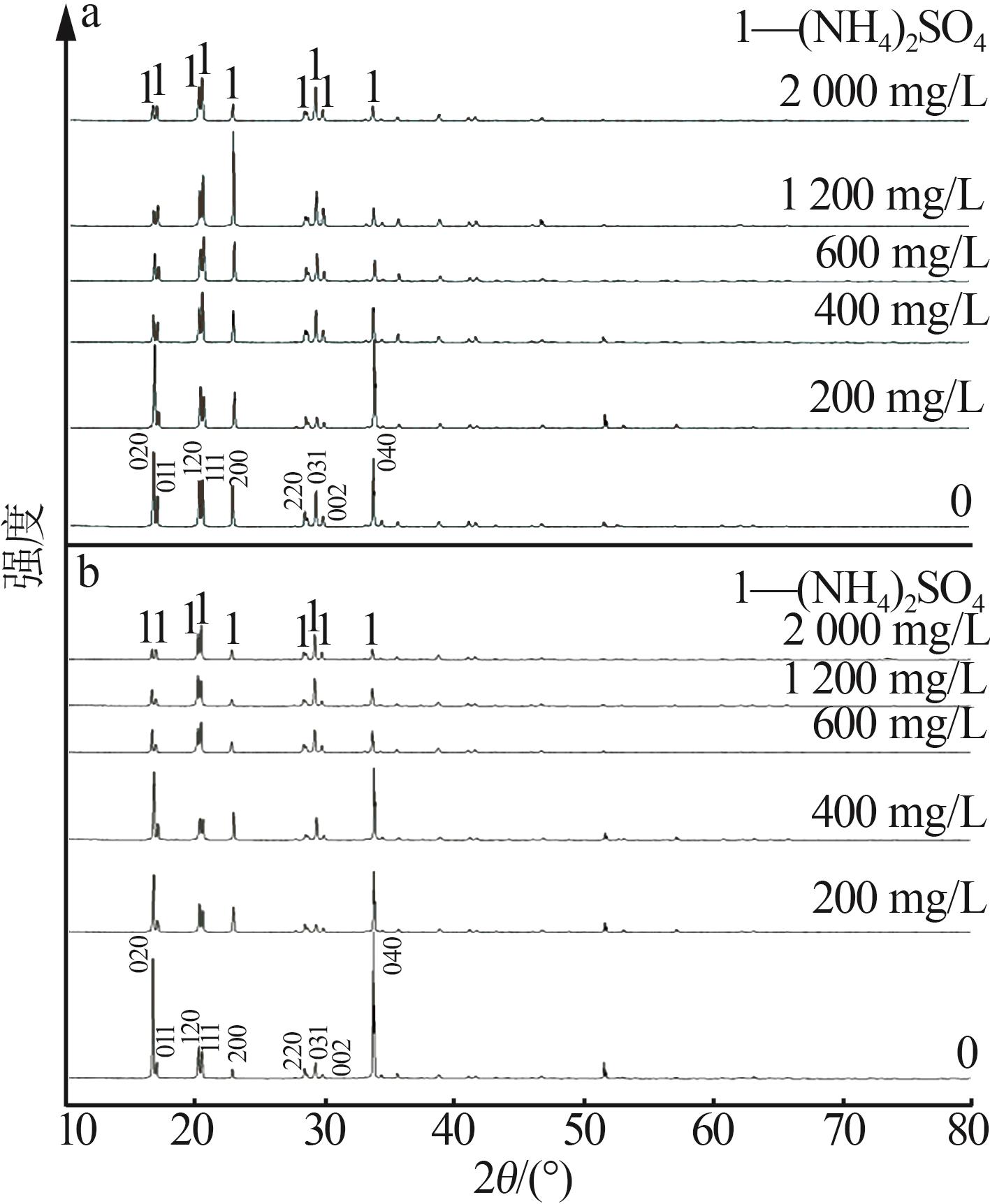
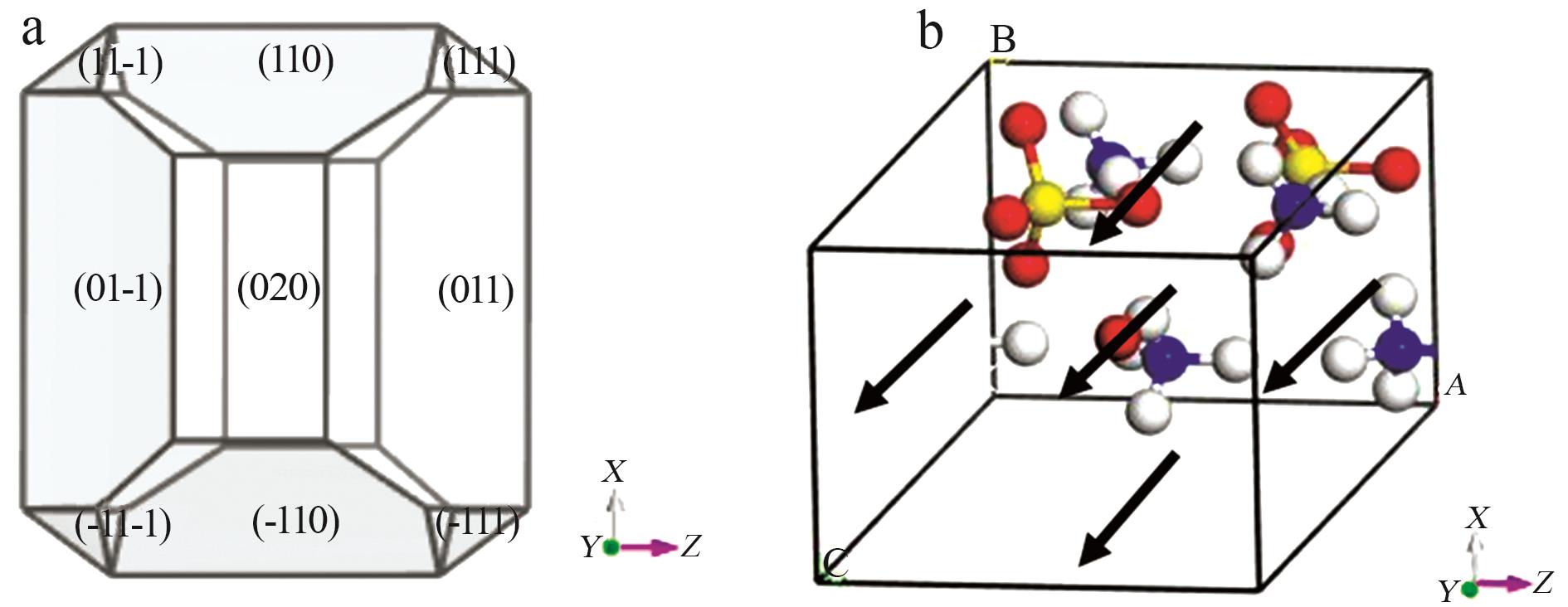
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (6): 27-34.doi: 10.19964/j.issn.1006-4990.2024-0364
• Research & Development • Previous Articles Next Articles
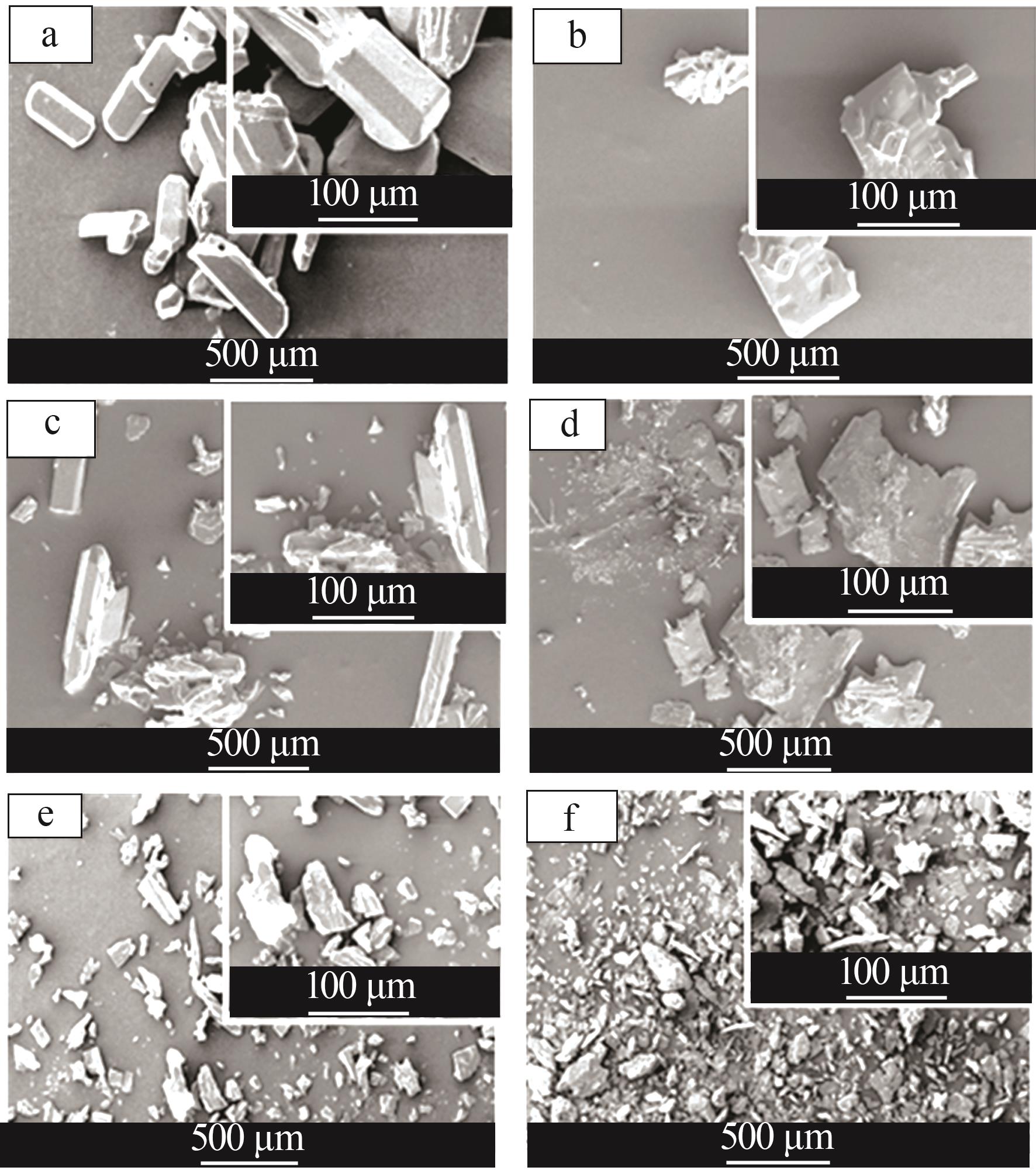
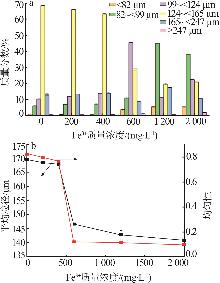
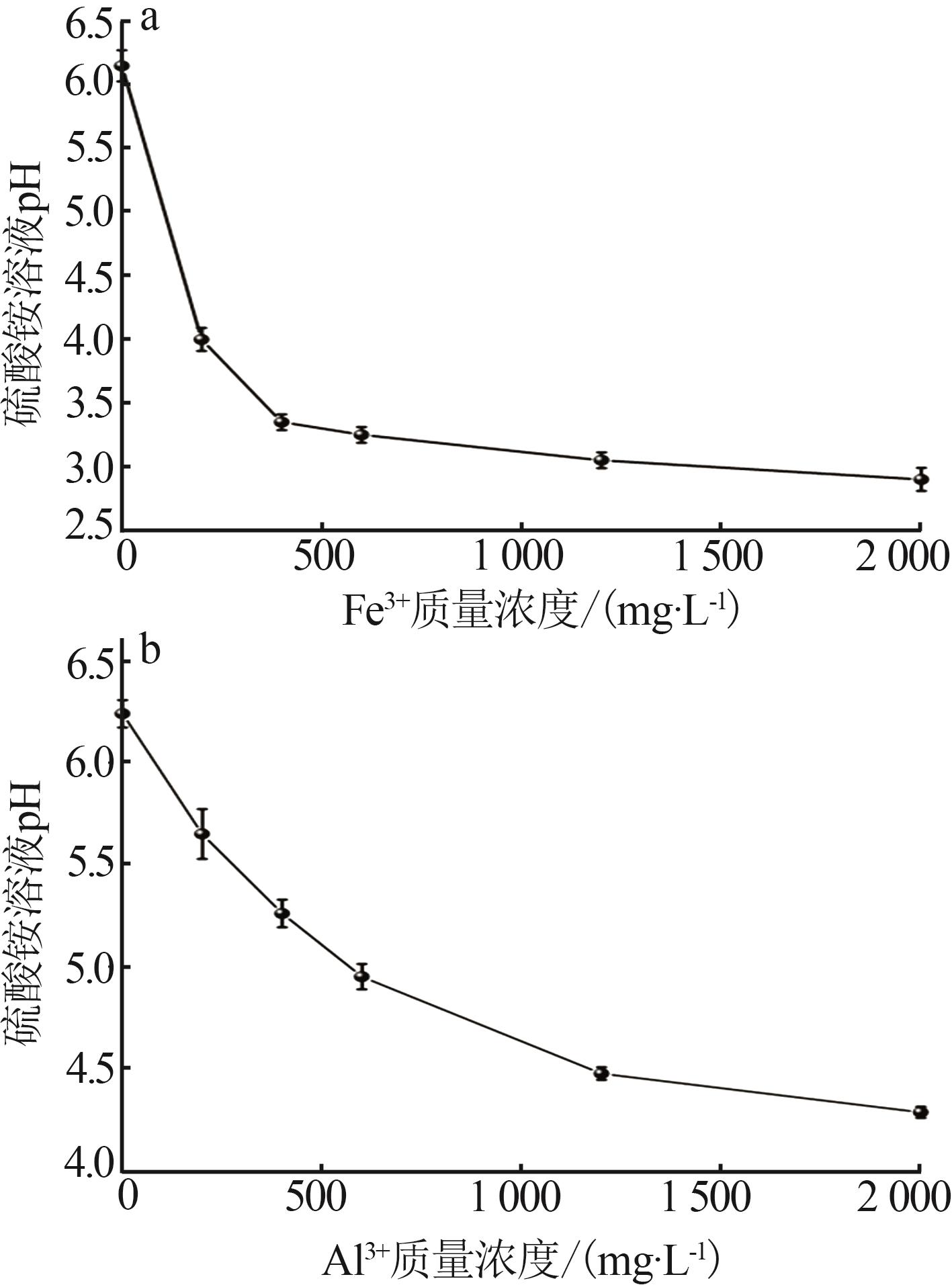
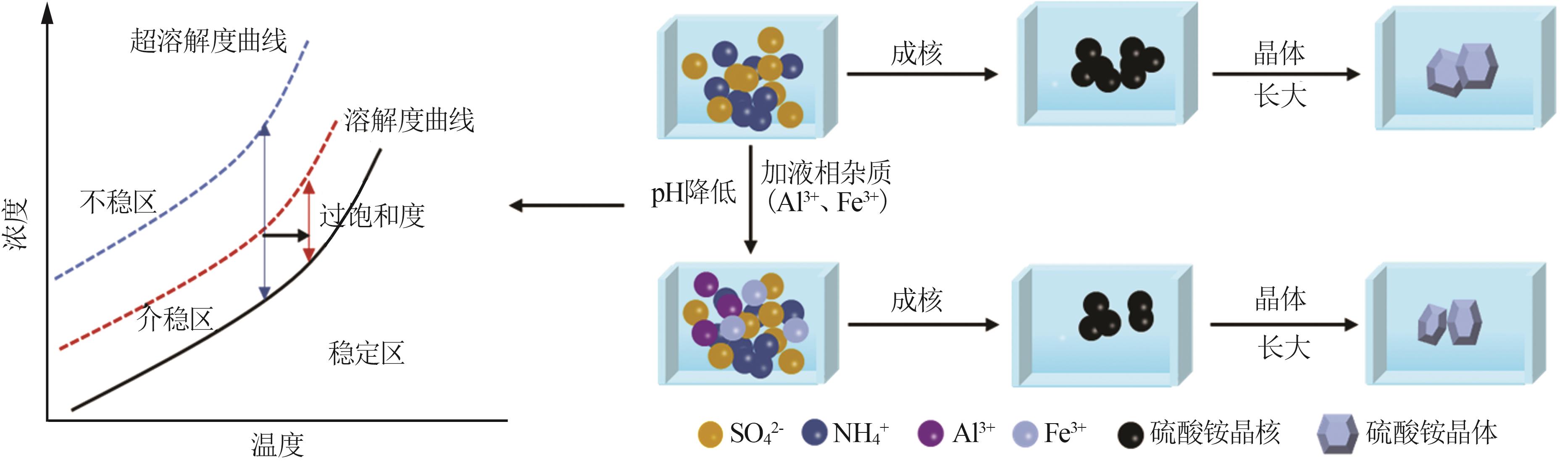
Effect of liquid phase impurity Fe and Al ions on crystallization of ammonium sulfate in ammonia flue gas desulphurization
XU Xiaojing1, ZHANG Yuanyuan1( ), LIU Wenqian1, LIU Shuyan1, WANG Fei1(
), LIU Wenqian1, LIU Shuyan1, WANG Fei1( ), GAO Yangyan1, YANG Yuhua2, YANG Fengling1
), GAO Yangyan1, YANG Yuhua2, YANG Fengling1
- 1. Engineering Research Center for CO2 Emission Reduction and Resource Utilization,Ministry of Education,Shanxi Yellow River Laboratory,Shanxi University,Taiyuan 030006,China
2. Solid Waste Comprehensive Utilization Changzhi(Xiangyuan) R&D Base of Shanxi University,Shanxi Xiangkuang Group Co. ,Ltd. ,Changzhi 046200,China
-
Received:2024-06-28Online:2025-06-10Published:2024-07-30 -
Contact:ZHANG Yuanyuan, WANG Fei E-mail:yuanyuanzhang@sxu.edu.cn;Wangfei1859@sxu.edu.cn
CLC Number:
Cite this article
XU Xiaojing, ZHANG Yuanyuan, LIU Wenqian, LIU Shuyan, WANG Fei, GAO Yangyan, YANG Yuhua, YANG Fengling. Effect of liquid phase impurity Fe and Al ions on crystallization of ammonium sulfate in ammonia flue gas desulphurization[J]. Inorganic Chemicals Industry, 2025, 57(6): 27-34.
share this article
| 1 | 荣维然.煤化工中氨法脱硫技术的应用研究[J].山西化工,2024,44(2):167-168. |
| RONG Weiran.Research on the application of ammonia desulfurization technology in coal chemical industry[J].Shanxi Chemical Industry,2024,44(2):167-168. | |
| 2 | 张全斌,周琼芳.中国燃煤烟气脱硫技术回顾与展望[J].科技创新与生产力,2020(5):27-32. |
| ZHANG Quanbin, ZHOU Qiongfang.Review and prospect of coal-fired flue gas desulfurization technology in China[J].Sci-Tech Innovation and Productivity,2020(5):27-32. | |
| 3 | 李露,黄帮福,张桂芳,等.氨法脱硫副产物硫酸铵蒸发结晶研究与进展[J].现代化工,2020,40(4):36-40. |
| LI Lu, HUANG Bangfu, ZHANG Guifang,et al.Research and advances in evaporation crystallization of ammonium sulfate byproduct from ammonia-route desulfurization[J].Modern Chemical Industry,2020,40(4):36-40. | |
| 4 | 陈欢哲,何海霞,万亚萌,等.燃煤烟气脱硫技术研究进展[J].无机盐工业,2019,51(5):6-11. |
| CHEN Huanzhe, HE Haixia, WAN Yameng,et al.Research progress on coal-fired flue gas desulfurization technology[J].Inorganic Chemicals Industry,2019,51(5):6-11. | |
| 5 | 董馨宇,闫双堆,闫秋艳,等.新型硫酸铵肥料对土壤团聚体碳、氮含量及玉米产量的影响[J].中国农业大学学报,2023,28(5):61-71. |
| DONG Xinyu, YAN Shuangdui, YAN Qiuyan,et al.Effects of new ammonium sulfate fertilizer on carbon and nitrogen content of soil aggregates and corn yield[J].Journal of China Agricultural University,2023,28(5):61-71. | |
| 6 | 刘伟,曾贞,刘亭.脱硫副产硫酸铵溶液在磷复肥生产中的应用[J].磷肥与复肥,2024,39(3):32-33,52. |
| LIU Wei, ZENG Zhen, LIU Ting.Application of ammonium sulfate solution produced during desulfurization in production of phosphate and compound fertilizer[J].Phosphate & Compound Fertilizer,2024,39(3):32-33,52. | |
| 7 | ZI Gaoyong, HUANG Bangfu, DONG Langshu,et al.Effect of evaporation temperature and Mg2+ concentration on the crystallization of ammonium sulfate[J].Crystal Research and Technology,2024,59(6):2300312. |
| 8 | WANG Lidong, WU Siyu, LIU Shuang,et al.Cobalt impregnated porous catalyst promoting ammonium sulfate recovery in an ammonia-based desulfurization process[J].Chemical Engineering Journal,2018,331:416-424. |
| 9 | 龙勇,韩永振.锅炉烟气氨法脱硫系统硫酸铵结晶影响因素分析[J].中氮肥,2023(6):69-73. |
| LONG Yong, HAN Yongzhen.Analysis of influencing factors of ammonium sulfate crystallization in ammonia desulfurization system of boiler flue gas[J].M-Sized Nitrogenous Fertilizer Progress,2023(6):69-73. | |
| 10 | FAN Xinchao, HUANG Bangfu, ZI Gaoyong,et al.Thermodynamic and kinetic study of (NH4)2SO4 crystallization in the presence of CaSO4 [J].Materials Today Communications,2024,39:108861. |
| 11 | PENG Jian, YANG Zhen, LIAN Peichao.Process optimization and crystallization kinetics of ammonium sulfate from wet ammonia-based desulfurization[J].Environmental Progress & Sustainable Energy,2024,43(2):e14310. |
| 12 | JIA Yong, YIN Liguo, XU Yalin,et al.A model for performance of sulfite oxidation of ammonia-based flue gas desulfurization system[J].Atmospheric Pollution Research,2015,6(6):997-1003. |
| 13 | 王振南,汪莉,李哲,等.Fe3+对氨法脱硫工艺中氧化和结晶的影响[J].环境工程,2015,33(S1):319-323,350. |
| WANG Zhennan, WANG Li, LI Zhe,et al.The impact of ferric iron on oxidation and crystallization in the ammonia desulfurization process[J].Engineering Environmental,2015,33(S1):319-323,350. | |
| 14 | 张军.溶液中离子对氨法脱硫结晶的影响[J].山西化工,2023,43(5):100-101,104. |
| ZHANG Jun.Effect of ions in solution on ammonia desulfurization crystallization[J].Shanxi Chemical Industry,2023,43(5):100-101,104. | |
| 15 | CAO Shaotao, ZHANG Yifei, ZHANG Yi.Nucleation and morphology of monosodium aluminate hydrate from concentrated sodium aluminate solutions[J].Crystal Growth & Design,2010,10(4):1605-1610. |
| 16 | 许士强.氨法脱硫的硫酸铵结晶影响因素及解决措施[J].云南化工,2018,45(5):87-88. |
| XU Shiqiang.Influencing factors and solutions of ammonium sulfate crystallization in ammonia desulfurization[J].Yunnan Chemical Technology,2018,45(5):87-88. | |
| 17 | 范嘉昊,张洋,范兵强,等.(NH4)2SO4和Na2SO4混合溶液中(NH4)2SO4结晶动力学及铁/铝/锰/铬等离子对(NH4)2SO4结晶的影响规律[J].化工进展,2023,42(1):488-496. |
| FAN Jiahao, ZHANG Yang, FAN Bingqiang,et al.Crystallization kinetics of (NH4)2SO4 in mixed solution of (NH4)2SO4 and Na2SO4 and the influence of Fe/Al/Mn/Cr ions on crystallization[J].Chemical Industry and Engineering Progress,2023,42(1):488- 496. | |
| 18 | HUANG Bangfu, DAI Meng, WEN Zhenjing,et al.Influence of ammonium nitrate on the crystallisation of ammonium sulfate[J].Environmental Technology,2024,45(11):2196-2204. |
| 19 | SHIM H M, KIM J W, KOO K K.Molecular interaction of solvent with crystal surfaces in the crystallization of ammonium sulfate[J].Journal of Crystal Growth,2013,373:64-68. |
| 20 | 张进华,樊小明,段付岗,等.氨法脱硫生产中硫酸铵结晶差的原因及工艺优化措施[J].中氮肥,2015(5):27-30. |
| ZHANG Jinhua, FAN Xiaoming, DUAN Fugang,et al.Causes of poor crystallization of ammonium sulfate in ammonia desulfurization production and process optimization measures[J].M-Sized Nitrogenous Fertilizer Progress,2015(5):27-30. | |
| 21 | ZI Gaoyong, HUANG Bangfu, LUO Liubin,et al.Optimization of ammonium sulfate crystallization under the action of ammonium chloride using the response surface method[J].Industrial & Engineering Chemistry Research,2023,62(48):20811-20820. |
| 22 | ARTEMOVA S, JAILLET L, REDON S.Automatic molecular structure perception for the universal force field[J].Journal of Computational Chemistry,2016,37(13):1191-1205. |
| 23 | YU Yaohong, BAI Jintao, MA Xiaohan,et al.Investigating the corrosive influence of chloride ions on slag recovery machine shells in power plants[J].Materials,2023,16(15):5270. |
| [1] | YU Hesong, WANG Dexi, YU Honglei, ZUO Maosheng, LI Jiazhi. Preparation of battery-grade iron phosphate by jet-enhanced air oxidation method [J]. Inorganic Chemicals Industry, 2025, 57(4): 31-36. |
| [2] | REN Genkuan, LUO Xin, ZHU Denglei, ZHANG Min. Thermodynamic analysis and experiment research on preparation of α-Fe2O3nanoparticles by solid-phase reduction method [J]. Inorganic Chemicals Industry, 2025, 57(4): 73-78. |
| [3] | WANG You, LIAO Lianzhen, CHEN Zheng, GAO Youjun. Effect of surfactants on electrocrystallization of Ni(OH)2 [J]. Inorganic Chemicals Industry, 2025, 57(3): 58-63. |
| [4] | LI Jiexuan, JIN Huiming. Study on preparation of Co-P-B/ZIF-67 catalyst and catalyzing hydrogen production from sodium borohydride hydrolysis [J]. Inorganic Chemicals Industry, 2024, 56(12): 150-158. |
| [5] | LI Ping, LI Jun, CHEN Ming. Study on process of recovery of Fe(Ⅲ) from spent lithium extractant and preparation of battery grade iron phosphate [J]. Inorganic Chemicals Industry, 2024, 56(10): 28-37. |
| [6] | WANG Junting, MA Hang, ZHA Zuotong, WAN Banglong, ZHANG Zhenhuan. Research progress of iron phosphate industrial wastewater treatment process [J]. Inorganic Chemicals Industry, 2024, 56(6): 26-33. |
| [7] | CHEN Yuneng, CHEN Kunfeng, XUE Dongfeng. Research progress of preparation and device application of lithium niobate crystal ferroelectric domain [J]. Inorganic Chemicals Industry, 2024, 56(6): 1-13. |
| [8] | LIU Hui, WANG Hongliang, YU Kun, GAO Shengnan. Effect of calcination on porous structure and electrochemical properties of air electrode [J]. Inorganic Chemicals Industry, 2024, 56(6): 80-86. |
| [9] | LI Kuai, LI Zhaoshuai, DONG Tingxuan, LI Dan, GUO Shengwei, HAN Fenglan. Study on effect of wet magnetic separation on distribution of Fe and heavy metal in fly ash [J]. Inorganic Chemicals Industry, 2024, 56(4): 98-104. |
| [10] | LIU Xiao, XIE Lei, DENG Jifeng, SHI Yu, ZHENG Jie. Study on performance of sponge supported Co-B catalyst for hydrogen generation from catalytic hydrolysis of sodium borohydride [J]. Inorganic Chemicals Industry, 2023, 55(12): 146-151. |
| [11] | WANG Zihan, LI Jun, CHEN Ming, ZHOU Qingyu. Study on preparation of battery grade ferric phosphate by co-precipitation of ferric nitrate and phosphoric acid [J]. Inorganic Chemicals Industry, 2023, 55(7): 51-57. |
| [12] | XU Li, ZHANG Qiang. Experimental study on properties of iron tailings powder cement-based materials [J]. Inorganic Chemicals Industry, 2023, 55(6): 116-123. |
| [13] | PAN Xiaoxiao, ZHUANG Shuxin, SUN Yuqing, SUN Gaoxing, REN Yan, JIANG Shengyu. Research progress of modified-LiFePO4 as cathode materials for lithium ion batteries [J]. Inorganic Chemicals Industry, 2023, 55(6): 18-26. |
| [14] | LIU Rui, GAO Wei, ZHANG Wenjing, AN Hongxue, LI Zaixing. Catalytic degradation of Rhodamine B by ferroferric oxide-loaded bacterial residue biochar [J]. Inorganic Chemicals Industry, 2023, 55(4): 111-119. |
| [15] | HU Luoxing,HUANG Qimao,QU Hongyou. Preparation of Fe3O4 from FeCl2 by-product of new process of titanium dioxide by hydrochloric acid [J]. Inorganic Chemicals Industry, 2023, 55(1): 118-123. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||