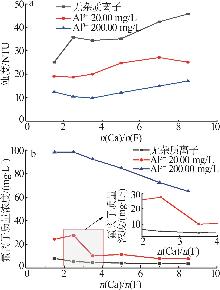
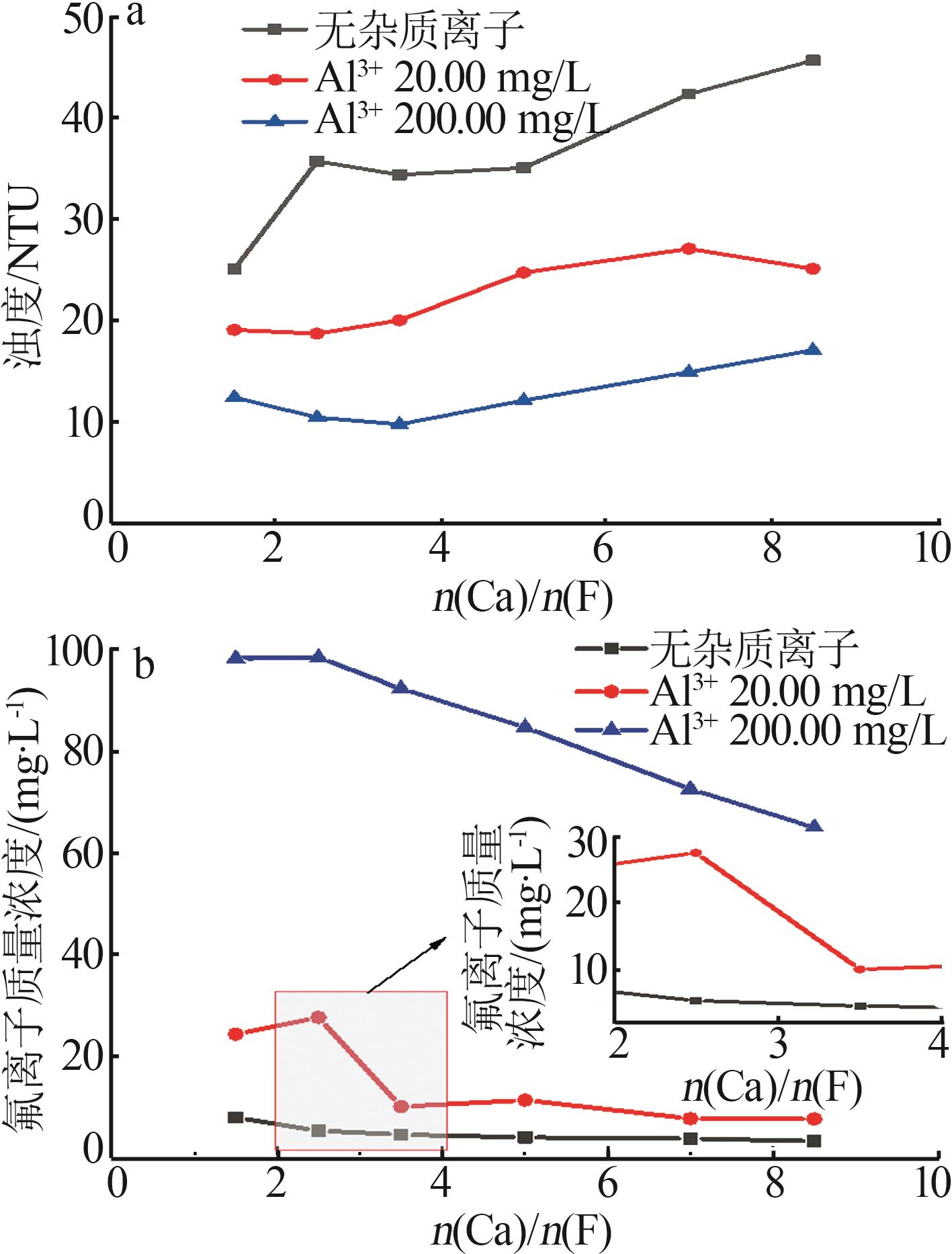
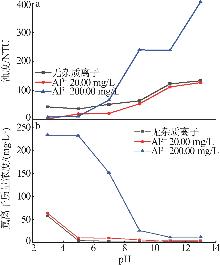
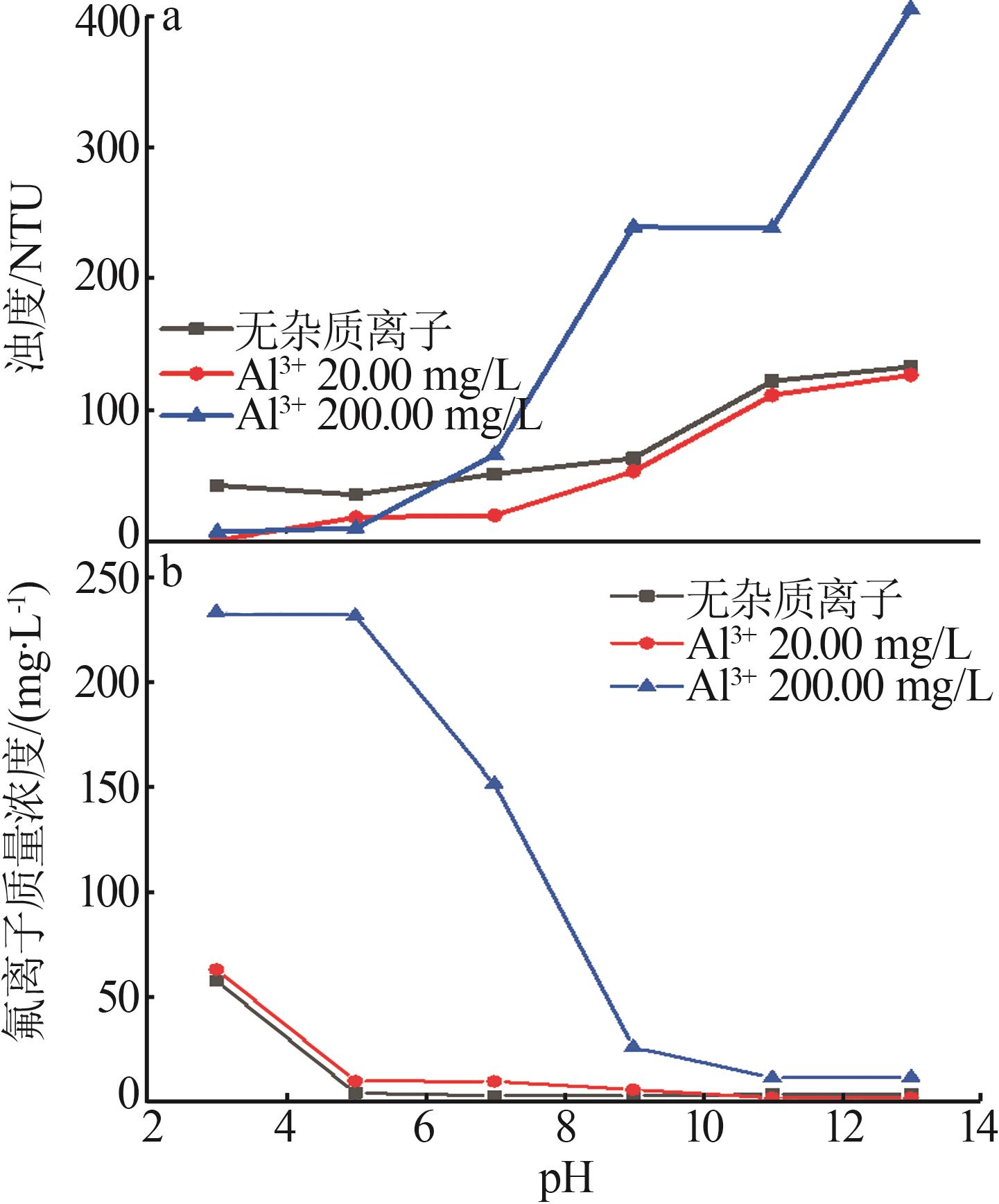
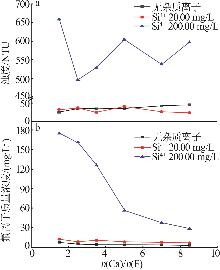
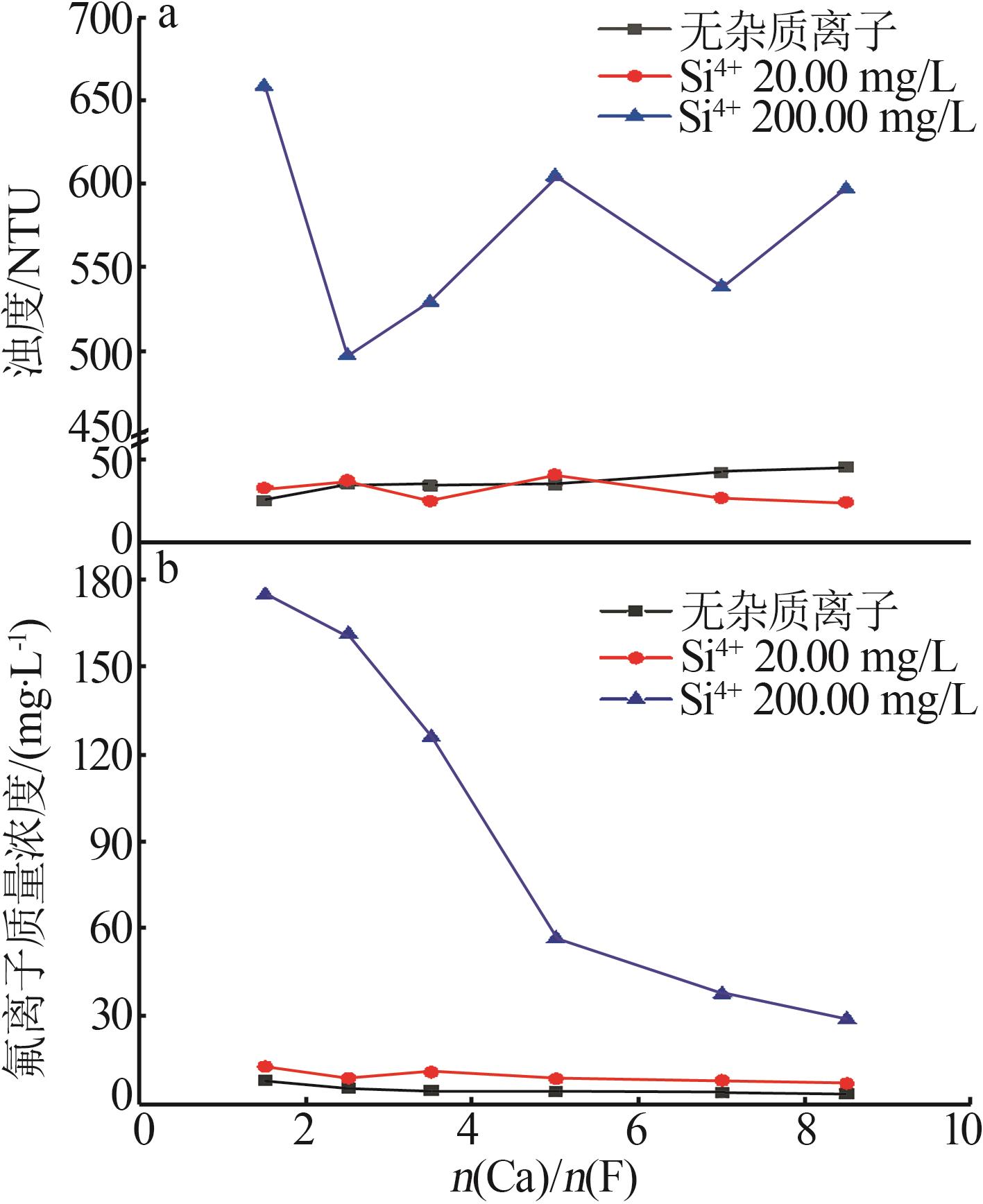
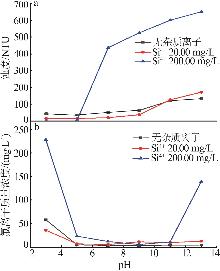
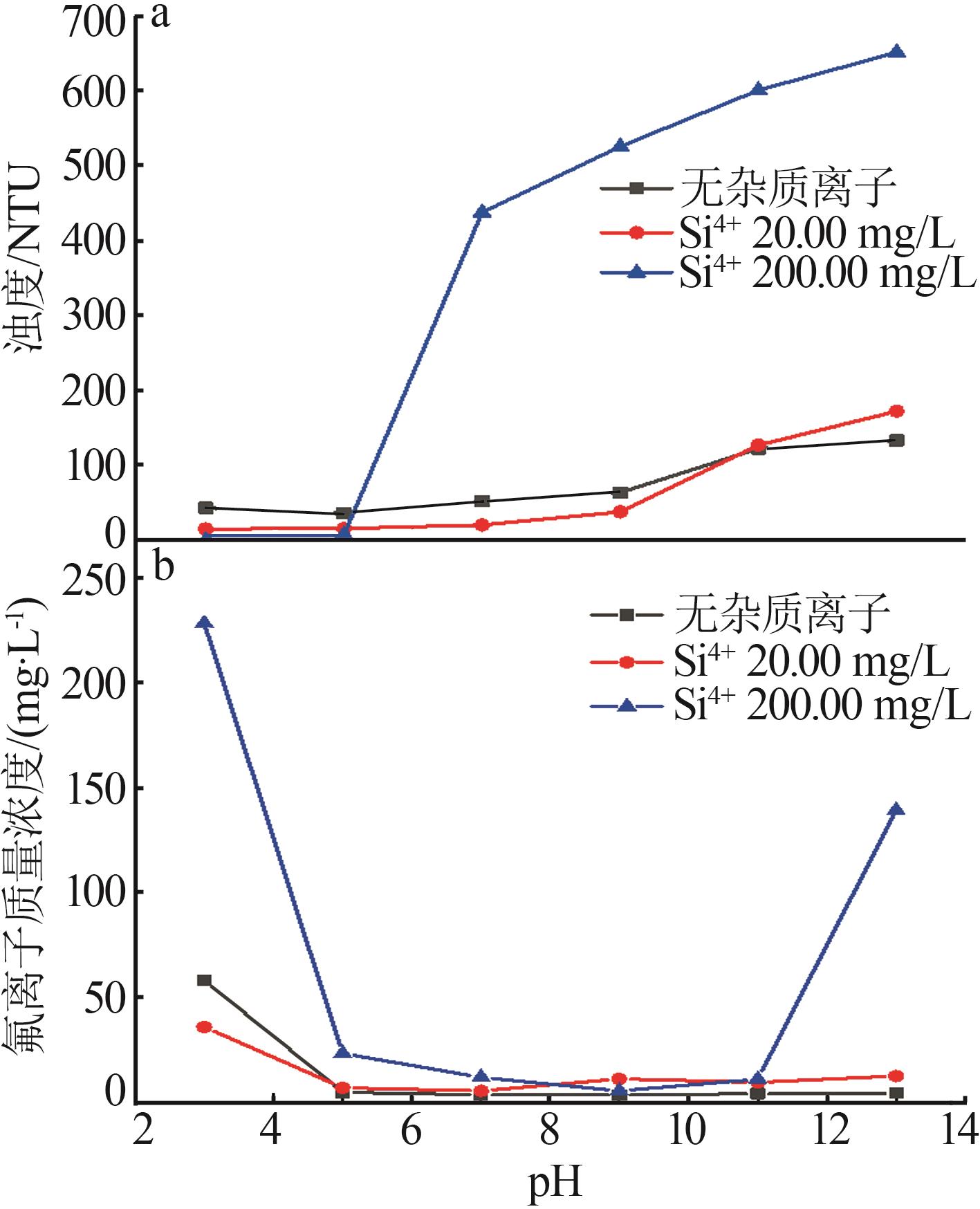
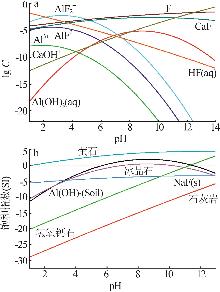
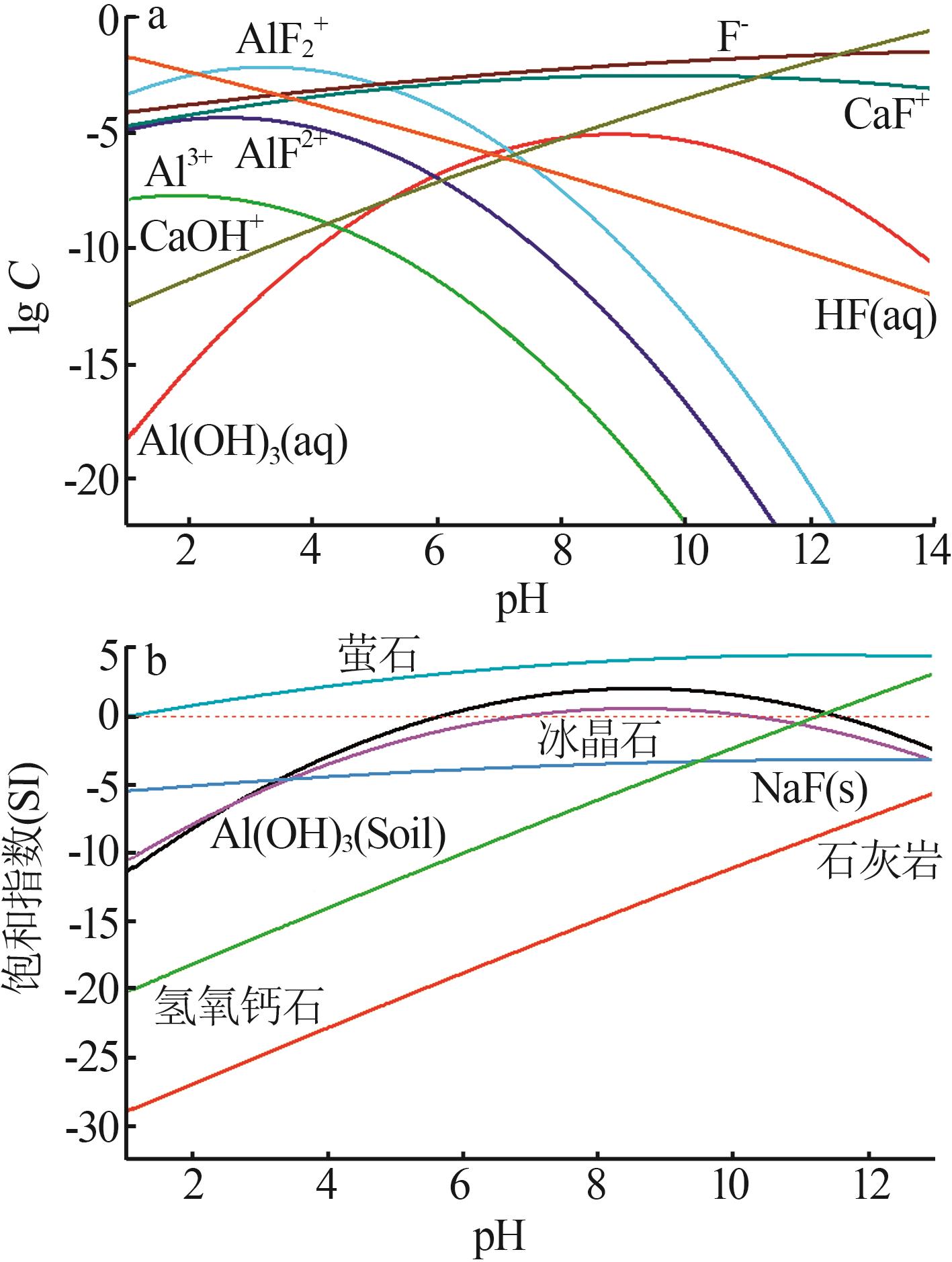
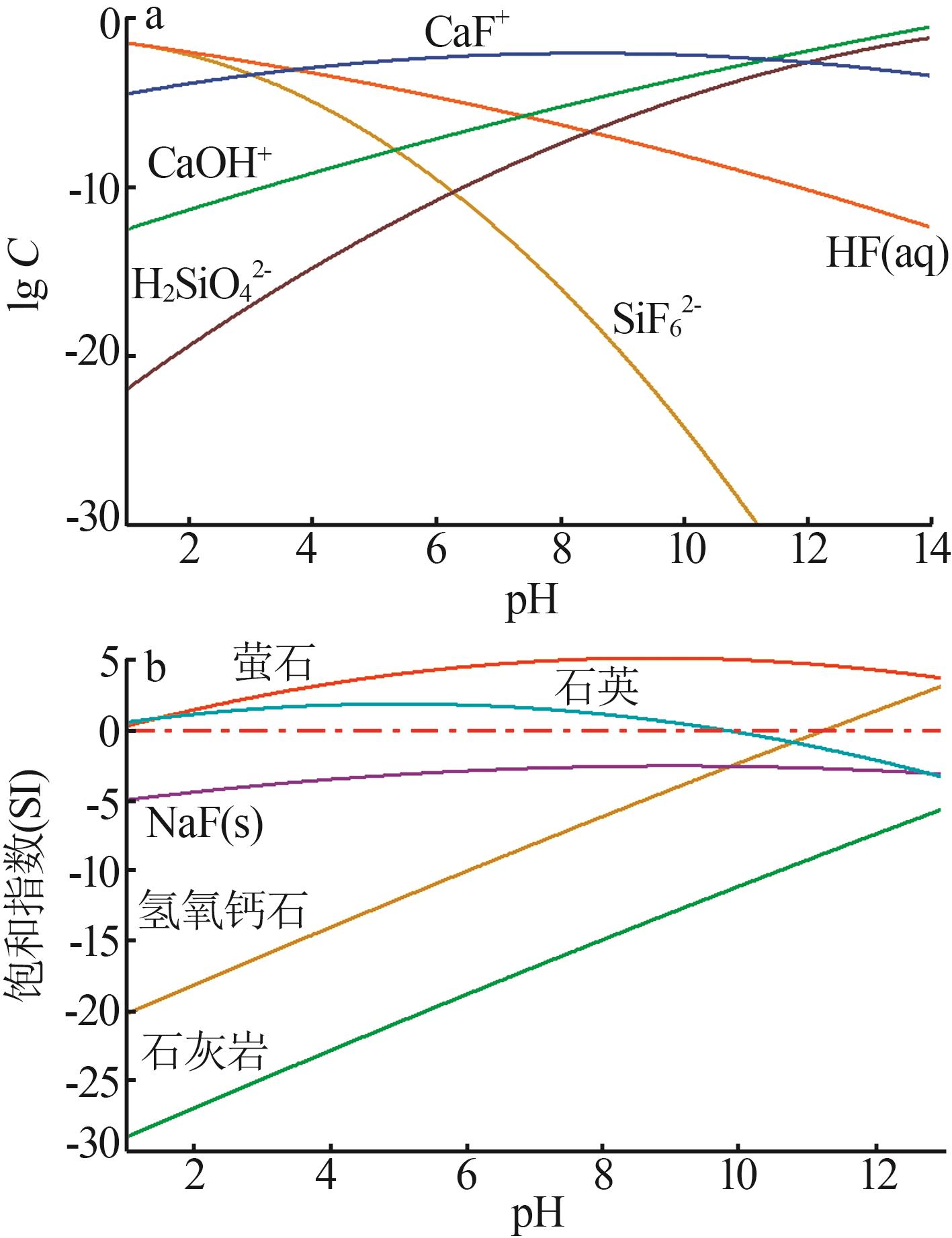
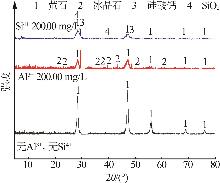
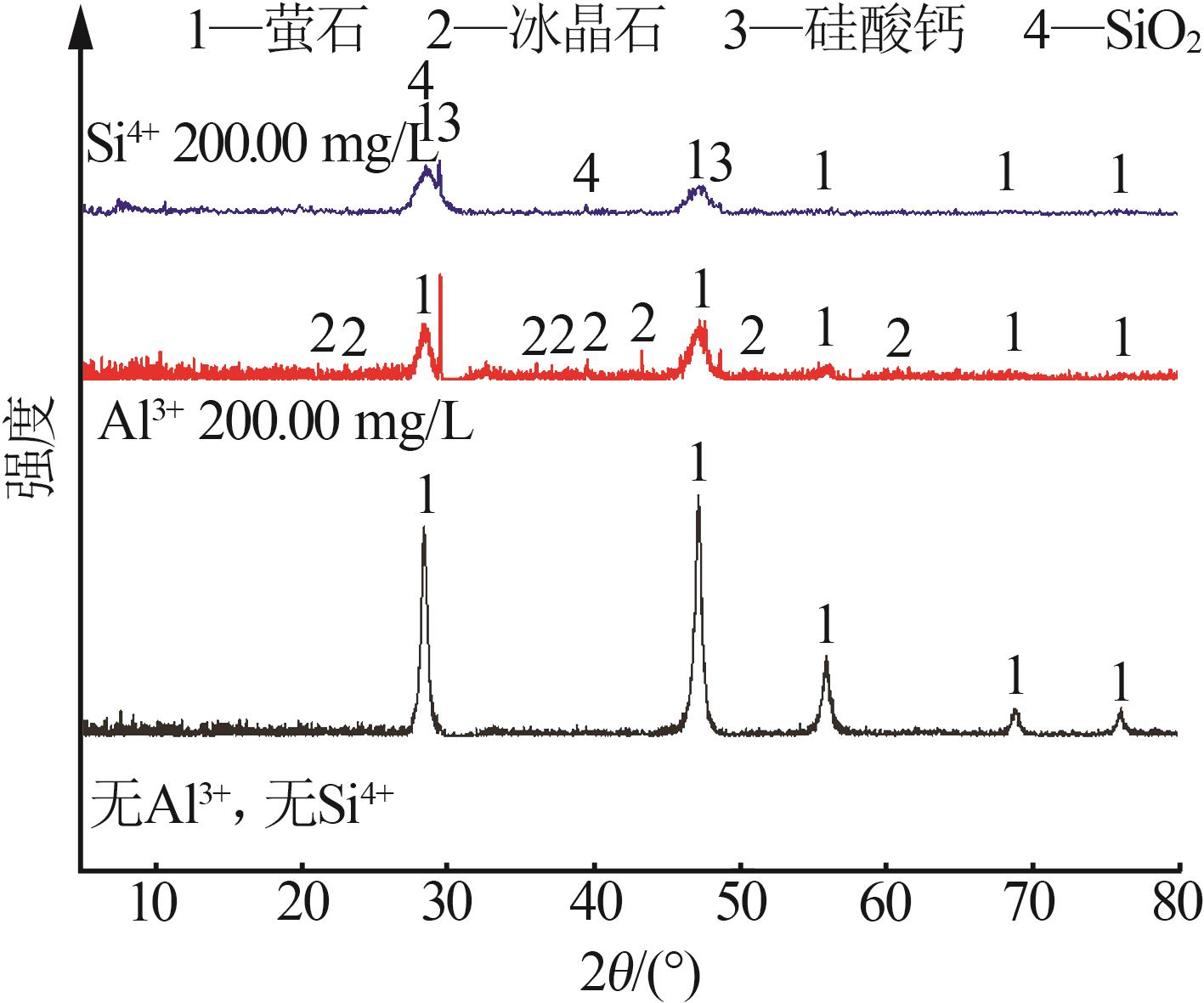
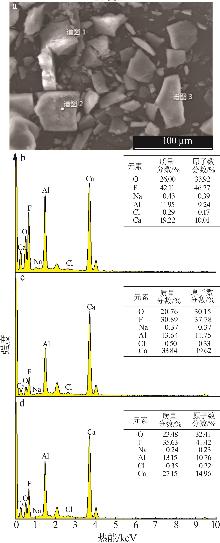
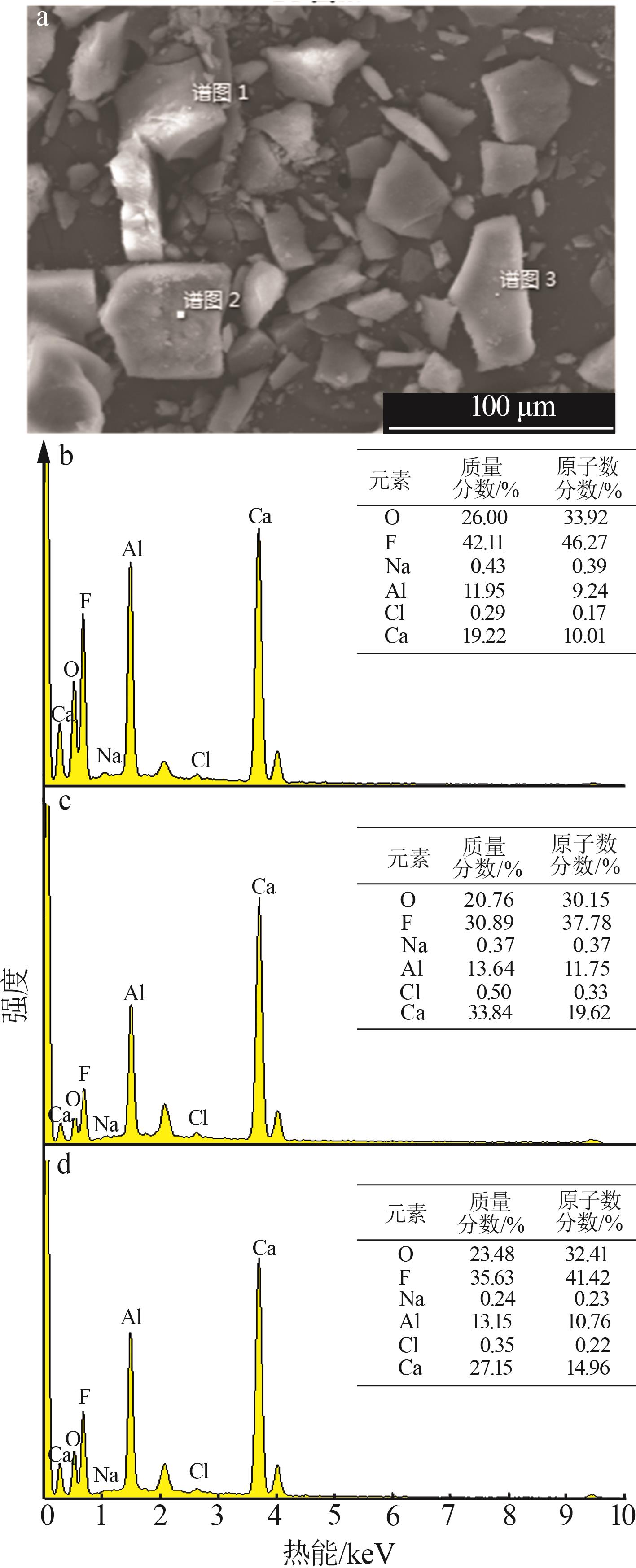
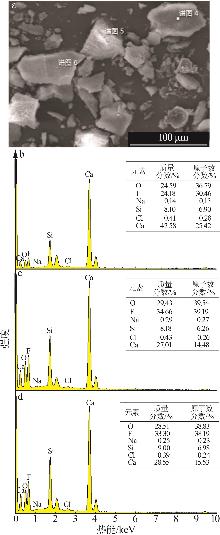
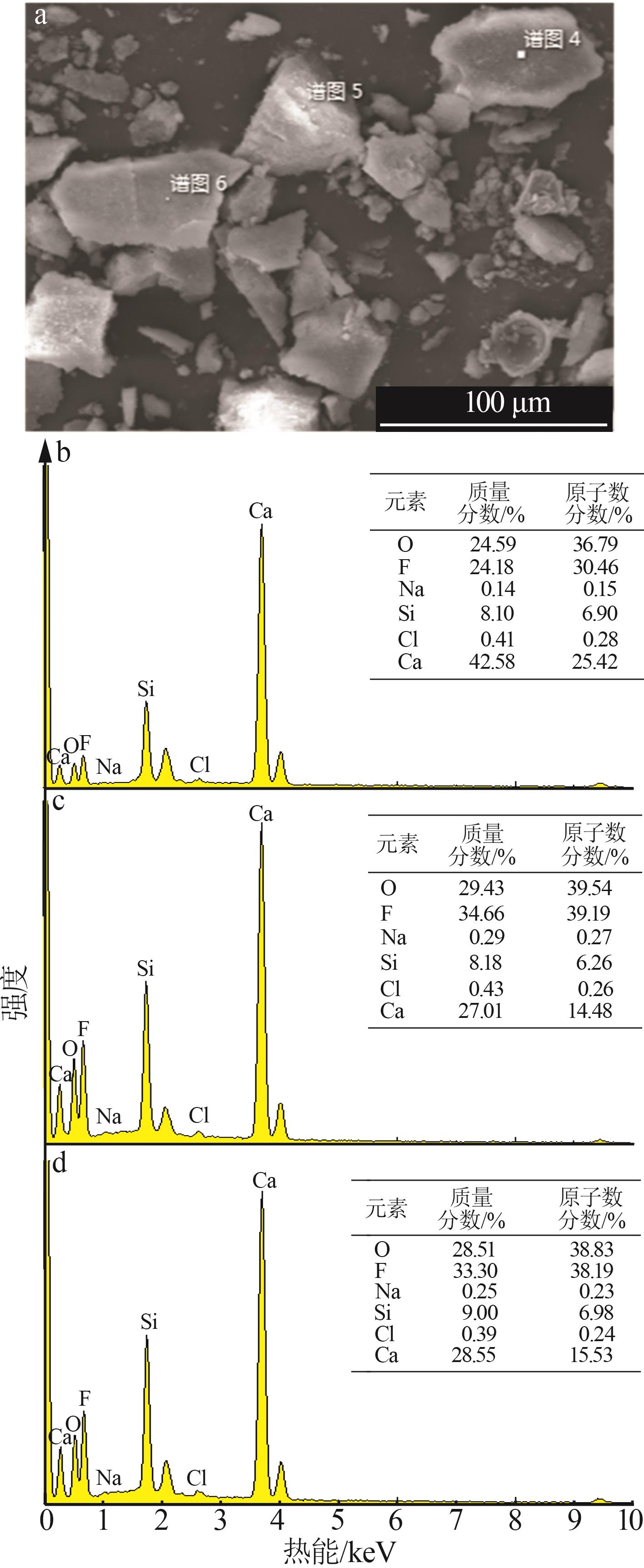
| [1] |
HORI H.Decomposition of fluorinated ionic liquids to fluoride ions using superheated water:An efficient approach for recovering fluorine from the waste of fluorinated ionic liquids[J].Electrochemistry,2021,89(2):75-82.
|
| [2] |
蒯杰,赵宇.含氟工业废水处理技术现状[J].资源节约与环保,2020(6):105-106.
|
|
KUAI Jie, ZHAO Yu.Present situation of fluorine-containing industrial wastewater treatment technology[J].Resources Economiza-tion & Environmental Protection,2020(6):105-106.
|
| [3] |
王小兵,曾佳玮,汤钟.高出水标准要求下高含氟工业废水处理实践[J].中国给水排水,2022,38(10):83-89.
|
|
WANG Xiaobing, ZENG Jiawei, TANG Zhong.Practice of high fluorine industrial wastewater treatment under the requirement of high effluent standard[J].China Water & Wastewater,2022,38(10):83-89.
|
| [4] |
高彦林,王金明,范永梅.高浓度含氟废水处理工程分析研究[J/OL].工业水处理,2024:1-11.Doi:10.19965/j.cnki.iwt.2024-0347.
|
|
GAO Yanlin, WANG Jinming, FAN Yongmei.Analysis and research of treatment project for high concentration fluorine-containing wastewater[J/OL].Industrial Water Treatment,2024:1-11.Doi:10.19965/j.cnki.iwt.2024-0347.
|
| [5] |
黄毅,李凯希,刘凯,等.富铝精炼渣处理高浓度模拟含氟废水的性能和机理研究[J].安全与环境学报,2024,24(5):1977-1984.
|
|
HUANG Yi, LI Kaixi, LIU Kai,et al.Study of performance and mechanism of treating high concentration stimulated fluoridecontaining wastewater with Al-rich refining slag[J].Journal of Safety and Environment,2024,24(5):1977-1984.
|
| [6] |
刘霄,杨艺琳,王鹏,等.不同功能性除氟吸附材料在饮用水及含氟废水中的研究进展[J].环境化学,2024,43(10):3247-3260.
|
|
LIU Xiao, YANG Yilin, WANG Peng,et al.Research progress of different functional defluorination adsorbent materials in drinking water and fluorinated wastewater[J].Environmental Chemistry,2024,43(10):3247-3260.
|
| [7] |
石智如,武海霞,吴德勇,等.化学-混凝沉淀法处理酸性高浓度含氟光伏废水[J].南京工业大学学报(自然科学版),2024,46(3):338-344.
|
|
SHI Zhiru, WU Haixia, WU Deyong,et al.Treatment of acidic and highly concentrated fluorine-containing photovoltaic wastewater by chemical coagulation-precipitation method[J].Journal of Nanjing Tech University(Natural Science Edition),2024,46(3):338-344.
|
| [8] |
朱祚峤,施梦圆,毛瑞,等.混凝沉淀法处理冶金含氟废水工艺研究[J].无机盐工业,2024,56(4):118-124.
|
|
ZHU Zuoqiao, SHI Mengyuan, MAO Rui,et al.Study on treatment process of metallurgical fluorine-containing wastewater by coagulant sedimentation method[J].Inorganic Chemicals Industry,2024,56(4):118-124.
|
| [9] |
张钰卿,刘佳,许兵,等.含氟废水处理中的除氟吸附技术研究进展[J].净水技术,2022,41(5):23-29,61.
|
|
ZHANG Yuqing, LIU Jia, XU Bing,et al.Research progress of adsorption technology for defluorination in fluoride-containing wastewater treatment[J].Water Purification Technology,2022,41(5):23-29,61.
|
| [10] |
EMAMJOMEH M M, SIVAKUMAR M.An empirical model for defluoridation by batch monopolar electrocoagulation/flotation (ECF) process[J].Journal of Hazardous Materials,2006,131(1/2/3):118-125.
|
| [11] |
EMAMJOMEH M M, SIVAKUMAR M, VARYANI A S.Analysis and the understanding of fluoride removal mechanisms by an electrocoagulation/flotation(ECF) process[J].Desalination,2011,275(1/2/3):102-106.
|
| [12] |
HU C Y, LO S L, KUAN W H,et al.Removal of fluoride from semiconductor wastewater by electrocoagulation-flotation[J].Water Research,2005,39(5):895-901.
|
| [13] |
程浩铭,张翠玲,任昊晔,等.化学沉淀法处理高氟废水的工艺条件优化[J].兰州交通大学学报,2018,37(5):80-84.
|
|
CHENG Haoming, ZHANG Cuiling, REN Haoye,et al.Optimization of the technological conditions for the treatment of high fluoride wastewater by the chemical precipitation method[J].Journal of Lanzhou Jiaotong University,2018,37(5):80-84.
|
| [14] |
曹明义,何国凯,汤丹平.多级中和沉淀法在钢铁酸洗废水中的除氟应用[J].冶金动力,2021,40(5):73-75.
|
|
CAO Mingyi, HE Guokai, TANG Danping.Application of multistage neutralization precipitation method in fluoride removal from steel pickling wastewater[J].Metallurgical Power,2021,40(5):73-75.
|
| [15] |
高学睿.石墨提纯工业酸性含氟废水的处理工艺研究[D].哈尔滨:哈尔滨工业大学,2017.
|
|
GAO Xuerui.Study on Treatment Technology of acid fluorine-containing wastewater in the graphite purification industry [D].Harbin:Harbin Institute of Technology,2017.
|
| [16] |
董光辉.含氟废水及污泥资源化利用技术研究与应用[D].南京:东南大学,2019.
|
|
DONG Guanghui.Research and application of fluorine-containing wastewater and sludge resource utilization technology [D].Nanjing:Southeast University,2019.
|
| [17] |
乔继扬,刘艳丽,刘峰彪,等.高密度底泥回流诱导结晶工艺处理石墨提纯废水[J].有色金属工程,2023,13(10):135- 141.
|
|
QIAO Jiyang, LIU Yanli, LIU Fengbiao,et al.Treatment of graphite purification wastewater by high density sediment reflux induced crystallization process[J].Nonferrous Metals Engineering,2023,13(10):135-141.
|
| [18] |
罗丽琼.基于铝工业含氟废水的除氟工艺研究[D].长沙:中南大学,2022.
|
|
LUO Liqiong.Fluoride removal process for fluoride wastewater from aluminum industry[D].Changsha:Central South University,2022.
|
| [19] |
FREE M L, MILLER J D.The significance of collector colloid adsorption phenomena in the fluorite/oleate flotation system as revealed by FTIR/IRS and solution chemistry analysis[J].International Journal of Mineral Processing,1996,48(3/4):197-216.
|
| [20] |
HUANG Haiming, LIU Jiahui, ZHANG Peng,et al.Investigation on the simultaneous removal of fluoride,ammonia nitrogen and phosphate from semiconductor wastewater using chemical precipitation[J].Chemical Engineering Journal,2017,307:696- 706.
|
| [21] |
何廷树,王敏豪,达永琪,等.含氟污泥的理化性质及其对水泥性能和环境安全性的影响[J].硅酸盐学报,2020,48(8):1295-1301.
|
|
HE Tingshu, WANG Minhao, Yongqi DA,et al.Physicochemical performance of fluorine-containing sludge and its effect on cement properties and environmental safety[J].Journal of the Chinese Ceramic Society,2020,48(8):1295-1301.
|
| [22] |
NTUKA U, TAITB S, WHITE E T.The precipitation and solubility of aluminum hydroxy fluoride hydrate between 30 and 70 ℃[J].Hydrometallurgy,2015,155:79-87.
|
| [23] |
LISBONA D F, SOMERFIELD C, STEEL K M.Leaching of spent pot-lining with aluminium nitrate and nitric acid:Effect of reaction conditions and thermodynamic modelling of solution speciation[J].Hydrometallurgy,2013,134-135:132-143.
|
| [24] |
蒋捷,沈艳,廖晓斌,等.太阳能光伏企业含氟废水中氟硅酸含量的测定[J].江西化工,2022,38(4):56-58.
|
|
JIANG Jie, SHEN Yan, LIAO Xiaobin,et al.The fluosilicic acid content determination in fluoride wastewater for solar photovoltaic enterprise[J].Jiangxi Chemical Industry,2022,38(4):56- 58.
|
| [25] |
吴名秀,曾悦,李云琴,等.Visual MINTEQ在水/土环境重金属形态研究中的应用与展望[J].福州大学学报(自然科学版),2023,51(4):574-581.
|
|
WU Mingxiu, ZENG Yue, LI Yunqin,et al.Bibliometric analysis of heavy metal forms research in water and soil environment based on Visual MINTEQ[J].Journal of Fuzhou University (Natural Science Edition),2023,51(4):574-581.
|
 ), HUANG Siyun1, XIE Lei1, ZHANG Yuxi1, QIAN Yupeng1,2,3(
), HUANG Siyun1, XIE Lei1, ZHANG Yuxi1, QIAN Yupeng1,2,3( )
)