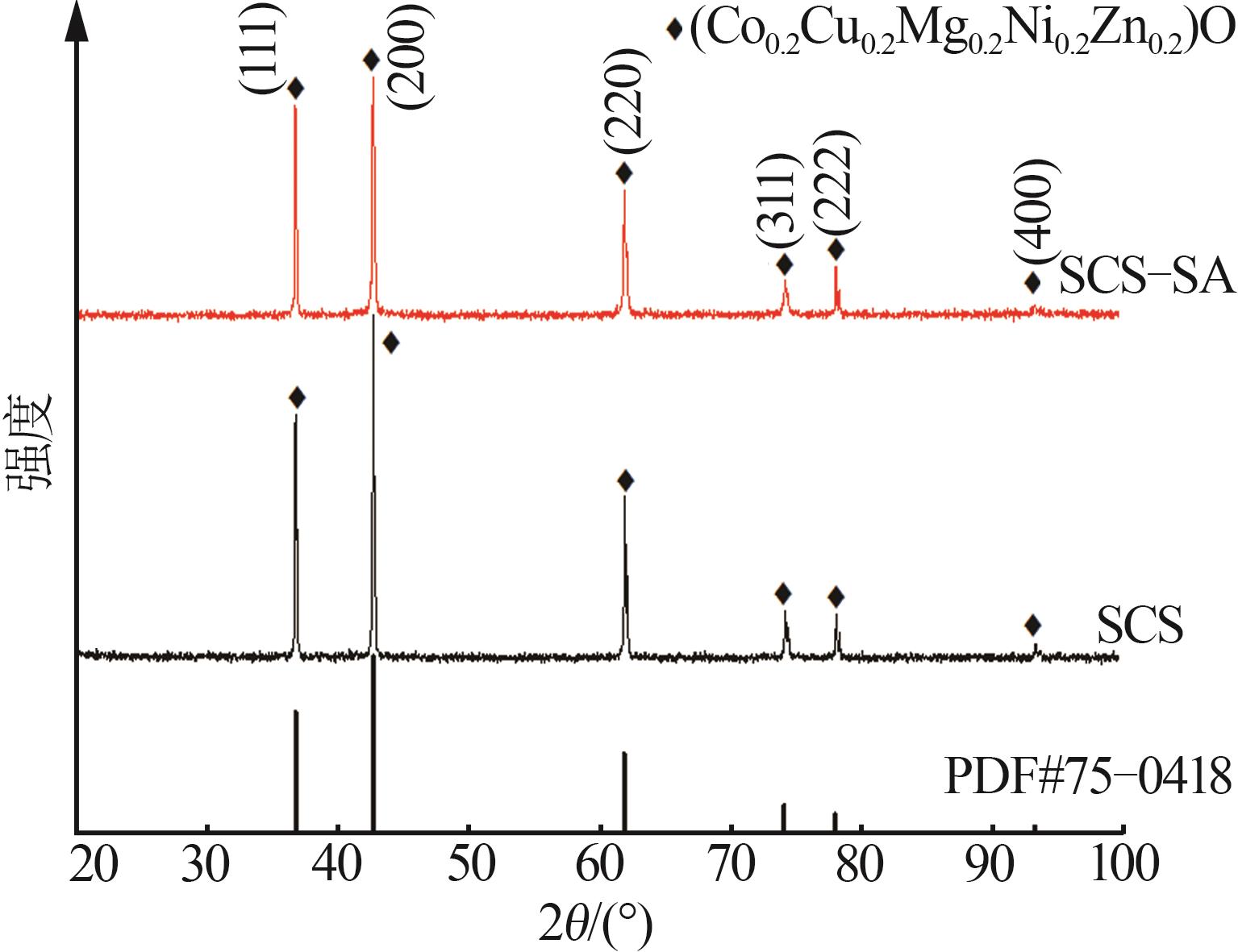
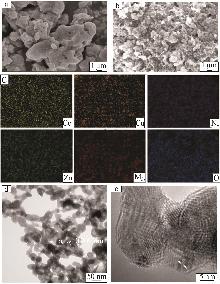
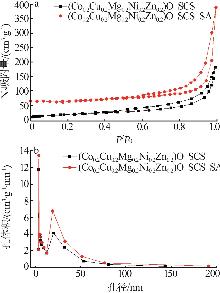
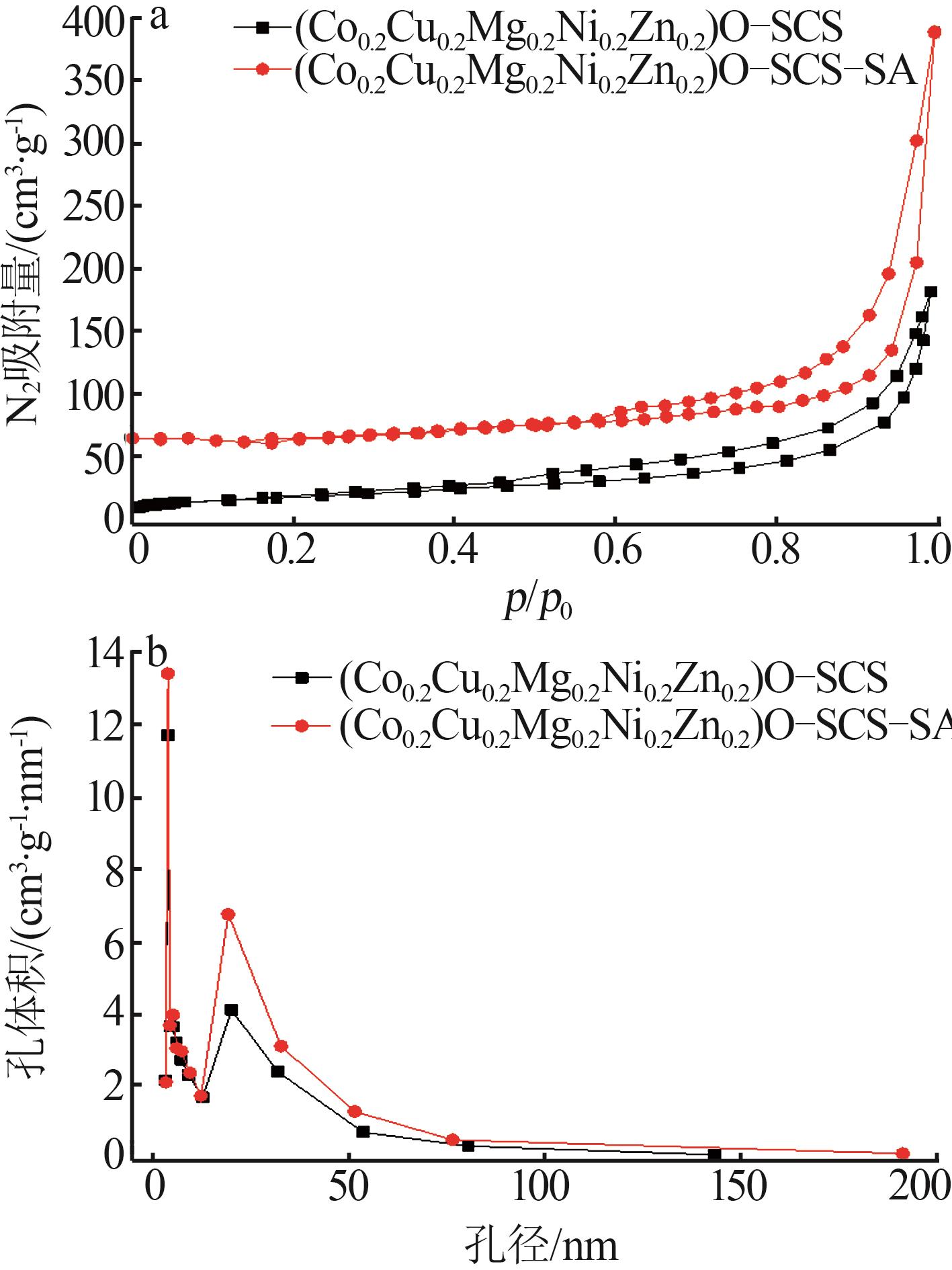
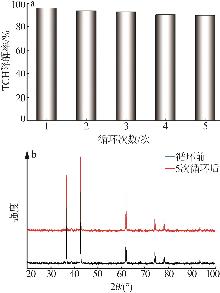
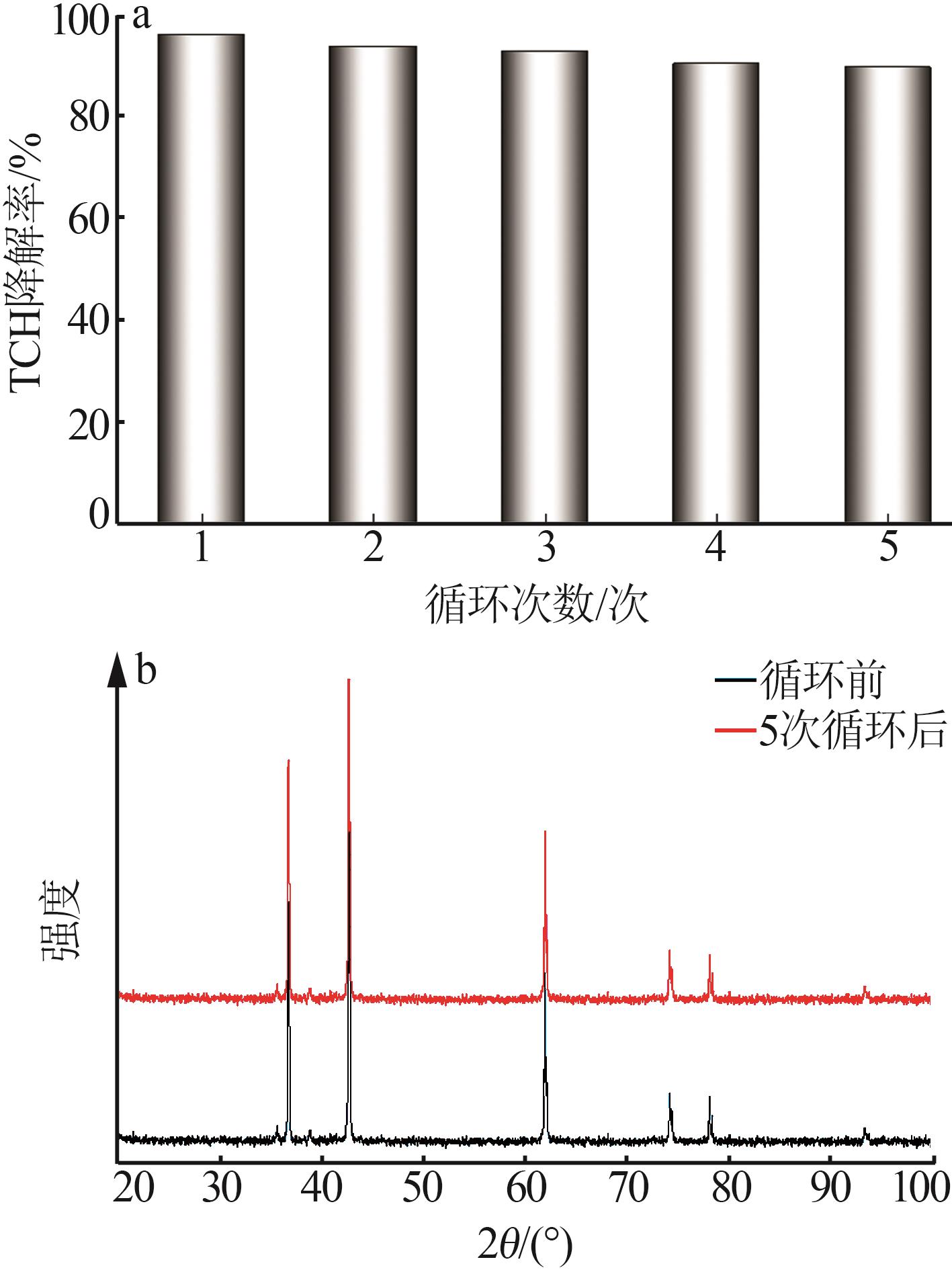
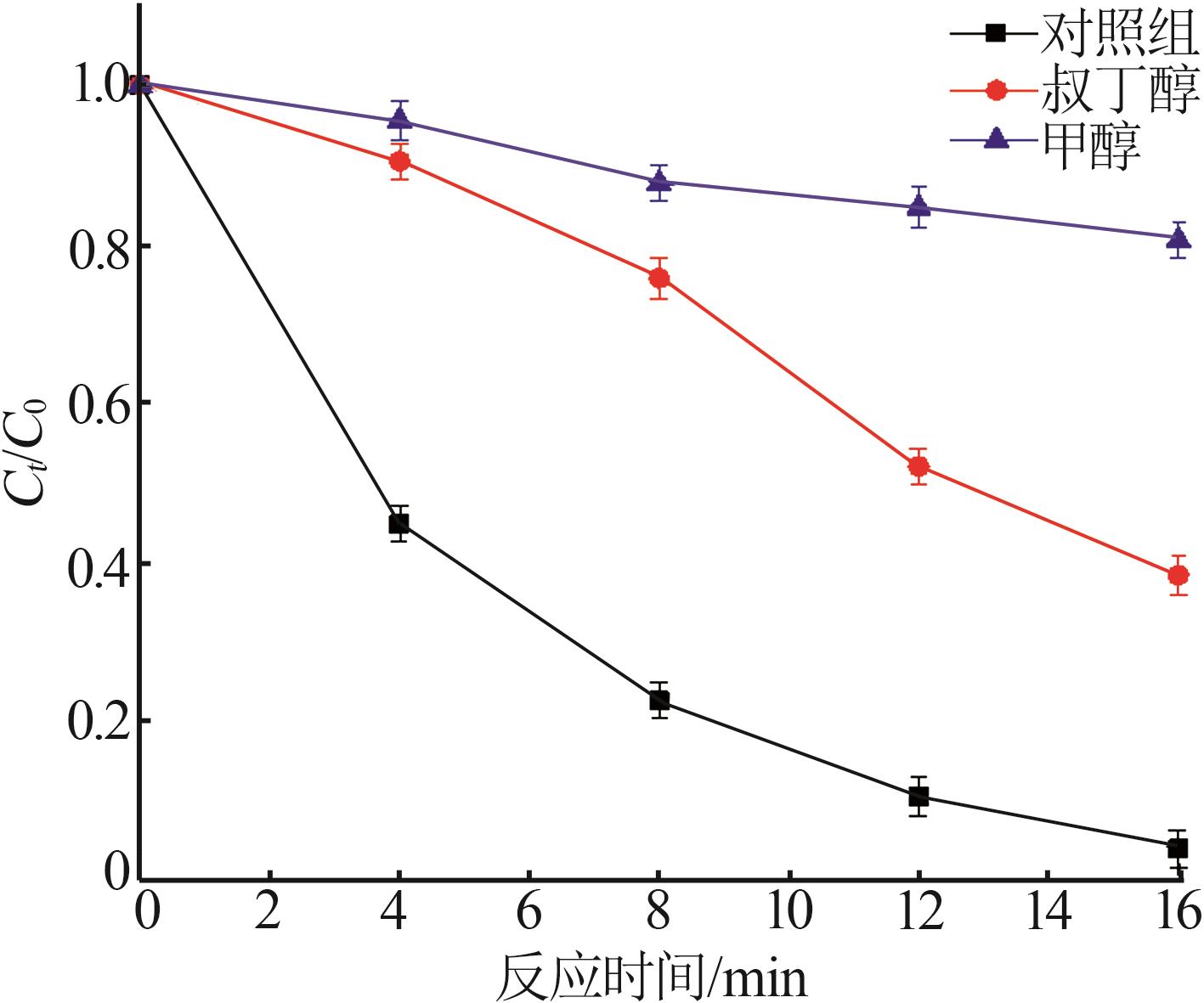
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (11): 115-122.doi: 10.19964/j.issn.1006-4990.2024-0559
• Catalytic Materials • Previous Articles Next Articles
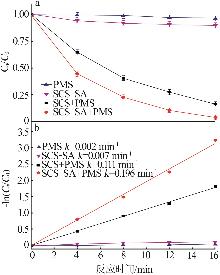
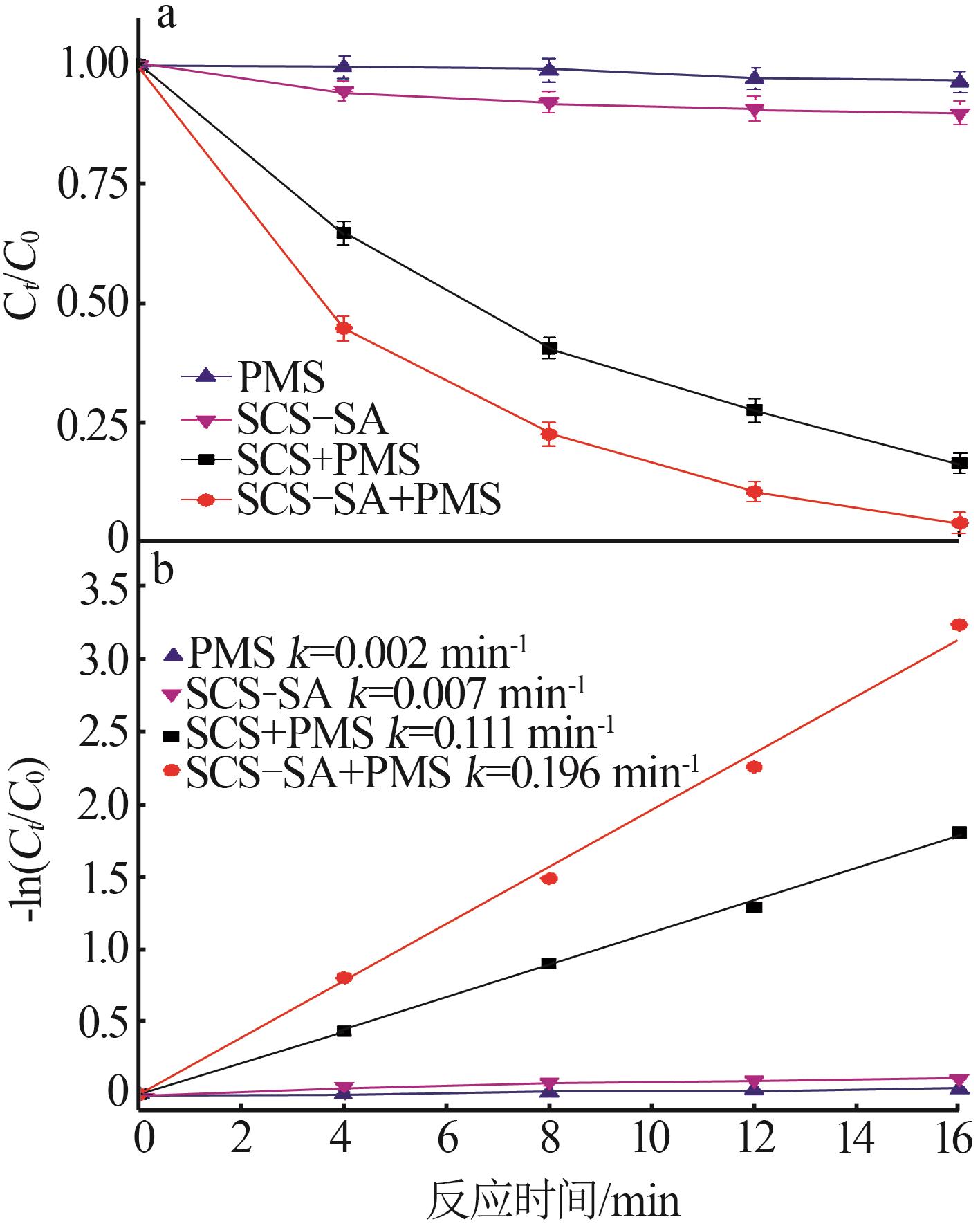
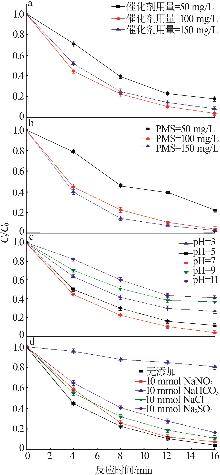
Study on degradation of tetracycline hydrochloride by high entropy oxide activated peroxymonsulfate
- School of Materials Science and Engineering,Chang′an University,Xi′an 710064,China
-
Received:2024-10-21Online:2025-11-10Published:2025-07-25
CLC Number:
Cite this article
YAN Xin, BAI Hongwei. Study on degradation of tetracycline hydrochloride by high entropy oxide activated peroxymonsulfate[J]. Inorganic Chemicals Industry, 2025, 57(11): 115-122.
share this article
| [1] | LIU Xiaohui, ZHANG Guodong, LIU Ying,et al.Occurrence and fate of antibiotics and antibiotic resistance genes in typical urban water of Beijing,China[J].Environment Pollution,2019,246 :163-173. |
| [2] | XU Longyao, ZHANG He, XIONG Ping,et al.Occurrence,fate,and risk assessment of typical tetracycline antibiotics in the aquatic environment:A review[J].Science of Total Environment,2021,753:141975. |
| [3] | GANIYU S O, SABLE S, EL-DIN M G.Advanced oxidation processes for the degradation of dissolved organics in produced water:A review of process performance,degradation kinetics and pathway[J].Chemical Engineering Journal,2022,429:132492. |
| [4] | WANG Jianlong, WANG Shizong.Activation of persulfate(PS) and peroxymonosulfate(PMS) and application for the degradation of emerging contaminants[J].Chemical Engineering Journal,2018,334 :1502-1517. |
| [5] | 张利洁,李德刚,韩文渊,等.磷掺杂碳量子点的制备及活化过一硫酸盐降解亚甲基蓝[J].无机盐工业,2024,56(01):126-133. |
| ZHANG Lijie, LI Degang, HAN Wenyuan,et al.Preparation of phosphorus-doped carbon quantum dots and activation of peroxymonosulfate for degradation of methylene blue[J].Inorganic Che-micals Industry,2024,56(1):126-133. | |
| [6] | WANG Debing, LIU Yun, WANG Qiaoying,et al.Activation of peroxydisulfate via photothermal synergistic strategy for wastewater treatment:Efficiency and mechanism[J].Journal of Hazardous Materials,2022,436:129224. |
| [7] | 韩伟,宋永明,刘琪,等.钙盐辅助热活化煤矸石及其活化PMS降解苯并[a]芘性能研究[J].无机盐工业,2025,57(1):103-112. |
| HAN Wei, SONG Yongming, LIU Qi,et al.Study on performance of calcium chloride assisted thermal activation of coal gangue and its peroxymonosulfate activation toward benzo(a)pyrene degradation[J].Inorganic Chemicals Industry,2025,57(01):103-112. | |
| [8] | CHERIFI Y, ADDAD A, VEZIN H,et al.PMS activation using reduced graphene oxide under sonication:efficient metal-free catalytic system for the degradation of rhodamine B,bisphenol A,and tetracycline[J].Ultrasonics Sonochemistry,2019,52:164-175. |
| [9] | HU Jiamin, ZHANG Jing, WANG Qingguo,et al.Efficient degradation of tetracycline by ultraviolet-based activation of peroxymonosulfate and persulfate[J].Water Science Technology,2019,79:911-920. |
| [10] | WANG Qiao, XU Zetao, WANG Songxue,et al.Rapid synthesis of amorphous CoO nanosheets:Highly efficient catalyst for parachlorophenol degradation by peroxymonosulfate activation[J].Separation and Purification Technology,2021,263:118369. |
| [11] | 夏强,廖小刚,李纲.化学浴沉积法制备氢氧化镍及其催化过一硫酸盐降解亚甲基蓝[J].无机盐工业,2021,53 (10):119-124. |
| XIA Qiang, LIAO Xiaogang, LI Gang.Degradation of methylene blue by catalytic peroxymonosulfate with Ni(OH)2synthesized through chemical bath deposition method[J].Inorganic Chemicals Industry,2021,53(10):119-124. | |
| [12] | 项伟,景燕娜,陈铮华,等.CuO/CN催化过一硫酸盐降解四环素性能及机理研究[J].无机盐工业,2024,56(8):123-130. |
| XIANG Wei, JING Yanna, CHEN Zhenghua,et al.Study on performance and mechanism of CuO/CN catalysed peroxynitrite degradation of tetracycline[J].Inorganic Chemicals Industry,2024,56(8):123-130. | |
| [13] | NI Tianjun, YANG Zhibin, ZHANG Hui,et al.Visible light assisted peroxymonosulfate activation by NiO/SnO2 composite for efficient tetracycline degradation[J].Applied Surface Science,2022,604:154537. |
| [14] | YANG Xue, WEI Gaoling, WU Puqiu,et al.Novel halloysite nanotube-based ultrafine CoMn2O4 catalyst for efficient degradation of pharmaceuticals through peroxymonosulfate activation[J].Applied Surface Science,2022,588:152899. |
| [15] | ZHENG Chaowen, NIU Huaiyuan, LIANG Chao,et al.A study on advanced oxidation mechanism of MnCo2O4/g-C3N4 degradation of nitrobenzene:sacrificial oxidation and radical oxidation[J].Chemical Engineering Journal,2021,403:126400. |
| [16] | HU Limin, ZHANG Guangshan, LIU Meng,et al.Application of nickel foam-supported Co3O4-Bi2O3 as a heterogeneous catalyst for BPA removal by peroxymonosulfate activation[J].Science of the Total Environment,2019,647:352-361. |
| [17] | SARKAR A, WANG Q S, SCHIELE A,et al.High-entropy oxides:Fundamental aspects and electrochemical properties[J].Advanced Materials,2019,31:1806236.. |
| [18] | YAN Xin, WANG Chaoli, AI Tao,et al.Synthesis of porous(Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O high entropy oxide catalysts for peroxymonosulfate activation toward tetracycline degradation[J].Inorganic Chemistry Communications,2023,150:110547. |
| [19] | ZUO Shiyu, LI Dongya, YANG Fan,et al.Copper oxide/graphitic carbon nitride composite for bisphenol a degradation by boosted peroxymonosulfate activation:Mechanism of Cu-O covalency governs[J].Journal of.Colloids.Interface.Science,2021,603:85-93. |
| [20] | YANG Qiangbin, AN Jibin, XU Zhonghao,et al.Performance and mechanism of atrazine degradation using Co3O4/g-C3N4 hybrid photocatalyst with peroxymonosulfate under visible light irradiation[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2021,614:126161. |
| [21] | DING Mingmei, AO Wang, XU Hang,et al.Facile construction of dual heterojunction CoO@TiO2/MXene hybrid with efficient and stable catalytic activity for phenol degradation with peroxymonosulfate under visible light irradiation[J].Journal of Hazardous Materials,2021,420:126686. |
| [22] | DONG Zhengtao, NIU Chenggang, GUO Hai,et al.Anchoring CuFe2O4 nanoparticles into N-doped carbon nanosheets for peroxymonosulfate activation:Built-in electric field dominated radical and non-radical process[J].Chemical Engineering Journal,2021,426:130850. |
| [23] | GUO Sheng, LIU Mengdie, YOU Liming,et al.Oxygen vacancy induced peroxymonosulfate activation by Mg-doped Fe2O3 composites for advanced oxidation of organic pollutants[J].Chemosphere,2021,279:130482. |
| [24] | YOU Junjie, SUN Weiyi, SU Shijun,et al.Degradation of bisphenol A by peroxymonosulfate activated with oxygen vacancy modified nano-NiO-ZnO composite oxides:A typical surface-bound radical system[J].Chemical Engineering Journal,2020,400:125915. |
| [25] | YAN Xin, QIN Jie, GAO Qiang,et al.Nano-sized ZnO supported on poly(triazineimide) nanotube for visible light drivenphotocatalytic reduction of Cr(Ⅵ)[J].Journal of Materials Science:Materials in Electronics,2018,29:19509-19516. |
| [26] | REN Fujun, WANG Tong, LIU Haitao,et al.CoMn2O4 nanoparticles embed in graphene oxide aerogel with three-dimensional network for practical application prospects of oxytetracycline degradation[J].Separation and Purification Technology,2021,259:118179. |
| [27] | YUAN Ruixia, JIANG Minglang, GAO Simeng,et al.3D mesoporous α-Co(OH)2 nanosheets electrodeposited on nickel foam:A new generation of macroscopic cobalt-based hybrid for peroxymonosulfate activation[J].Chemical Engineering Journal,2020,380:122447. |
| [28] | FAN Yanan, MA Wenjie, HE Jianglong,et al.CoMoO4 as a novel heterogeneous catalyst of peroxymonosulfate activation for the degradation of organic dyes[J].RSC Advances,2017,7:36193-36200. |
| [29] | XU Lijian, CHU Wei, GAN Lu.Environmental application of graphene-based CoFe2O4 as an activator of peroxymonosulfate for the degradation of a plasticizer[J].Chemical Engineering Journal,2015,263:435-443. |
| [30] | 黄艳,邢波,杨郭,等.钢渣/活性炭复合材料催化过硫酸盐降解盐酸四环素[J].工业水处理,2022,42 (3):131-137. |
| HUANG Yan, XING Bo, YANG Guo,et al.Catalytic degradation of tetracycline hydrochloride with persulfate catalyzed by composite of carbon and steel slag[J].Industrial Water Treatment,2022,42 (3):131-137.. | |
| [31] | 王渊源,阎鑫,艾涛,等.碳化泡沫负载Co3O4催化过硫酸盐降解罗丹明B[J].环境科学,2022,43(4):2039-2046. |
| WANG Yuanyuan, YAN Xin, AI Tao,et al.Carbonized foram supported Co3O4 activated peroxymonosulfate towards Rhodamine B degradation[J].Environmental Science,2022,43(4):2039-2046. | |
| [32] | PENG Lin, GONG Xiaobo, WANG Xinghong,et al.In situ growth of ZIF-67 on a nickel foam as a three-dimensional heterogeneous catalyst for peroxymonosulfate activation[J].RSC Advances,2018,8:26377-26382 |
| [33] | LI Xianghui, GUO Weilin, LIU Zhonghua,et al.Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation[J].Applied Surface Science,2016,369:130-136. |
| [1] | HAN Wei, SONG Yongming, LIU Qi, XU Jinling, XU Rong, LI Chunquan, YIN Shuaijun, SUN Zhiming. Study on performance of calcium chloride assisted thermal activation of coal gangue and its peroxymonosulfate activation toward benzo(a)pyrene degradation [J]. Inorganic Chemicals Industry, 2025, 57(1): 103-112. |
| [2] | ZUO Guangling, WANG Minghui, PENG Yunying, DU Jia, YE Hongyong. Study on hnoneycomb-like LaVO4/Bi2O3 heterojunction for photocatalytic degradation of tetracycline hydrochloride [J]. Inorganic Chemicals Industry, 2024, 56(11): 158-164. |
| [3] | YAN Chaoqun, ZHANG Xianming, WEI Juan, CHENG Zhiliang, XU Qian, ZHANG Xuan. Synthesis of cubic α-Fe2O3 catalyst and its photo-Fenton degradation performance of antibiotic under visible light [J]. Inorganic Chemicals Industry, 2023, 55(8): 28-35. |
| [4] | HUANG Xiyao,LI Mingchun,GUO Yintong. Study on synergistic modification of g-C3N4/Bi/Bi2WO6 photocatalyst [J]. Inorganic Chemicals Industry, 2022, 54(12): 133-138. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||