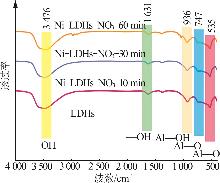
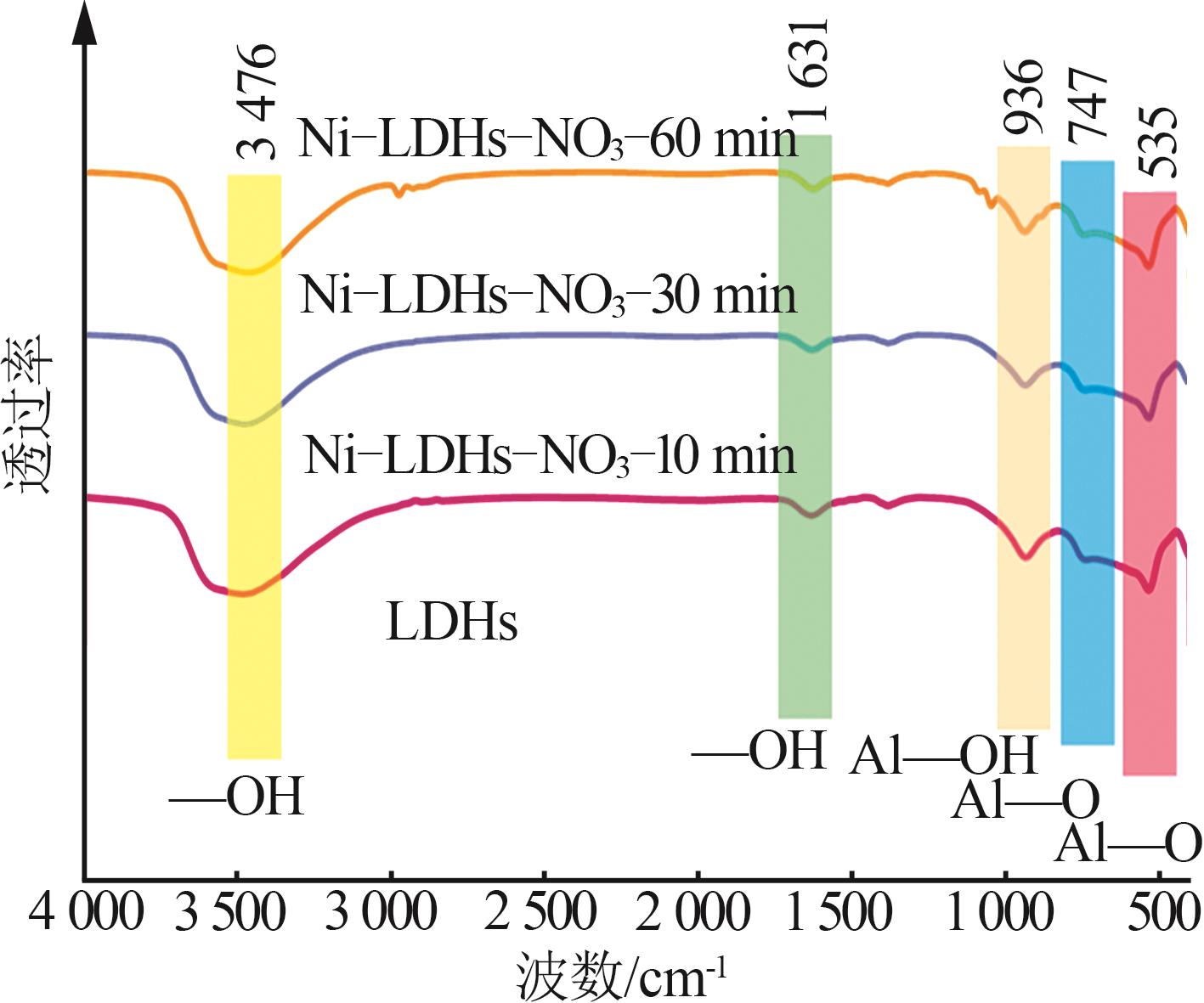
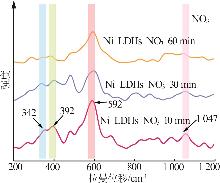
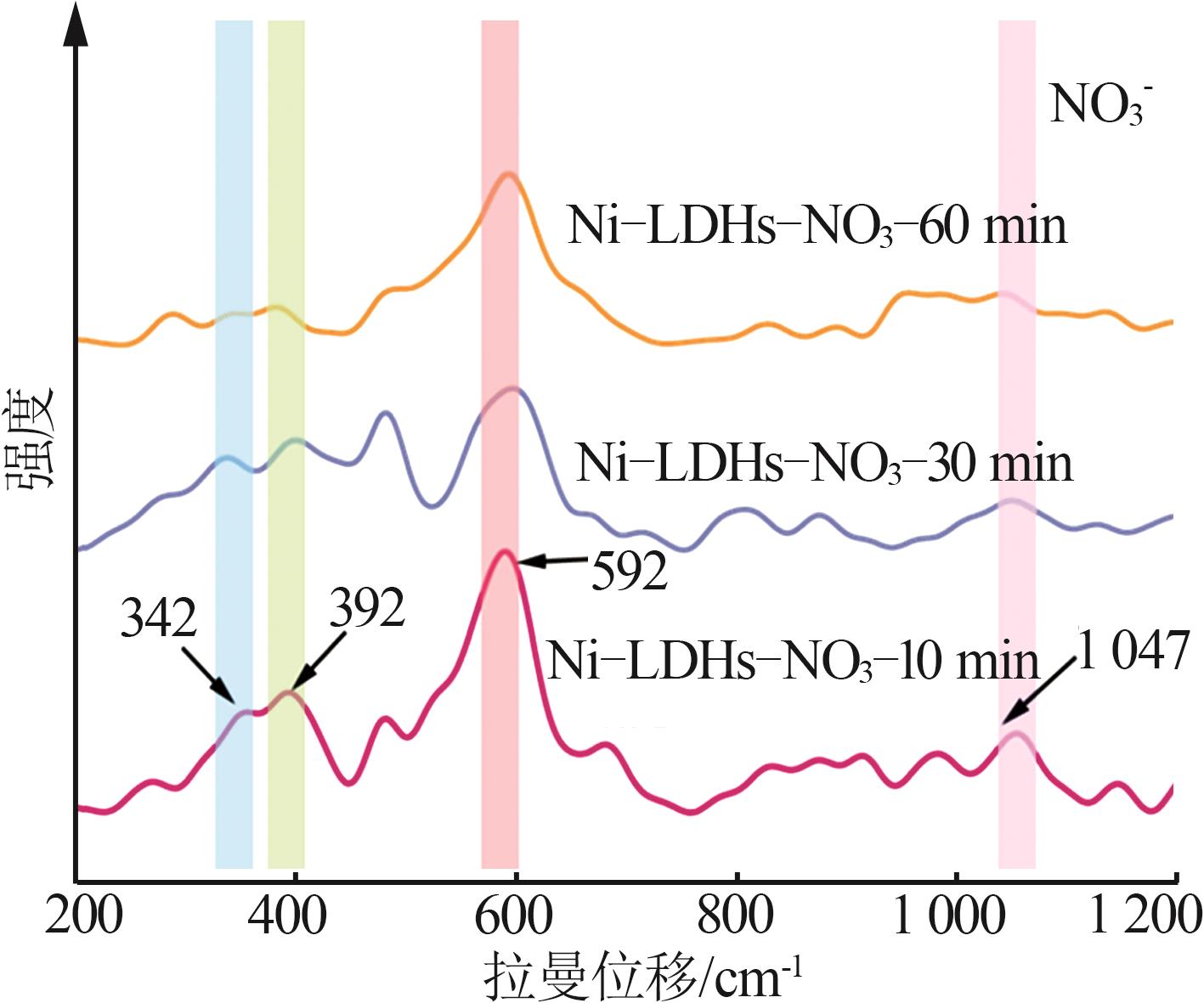
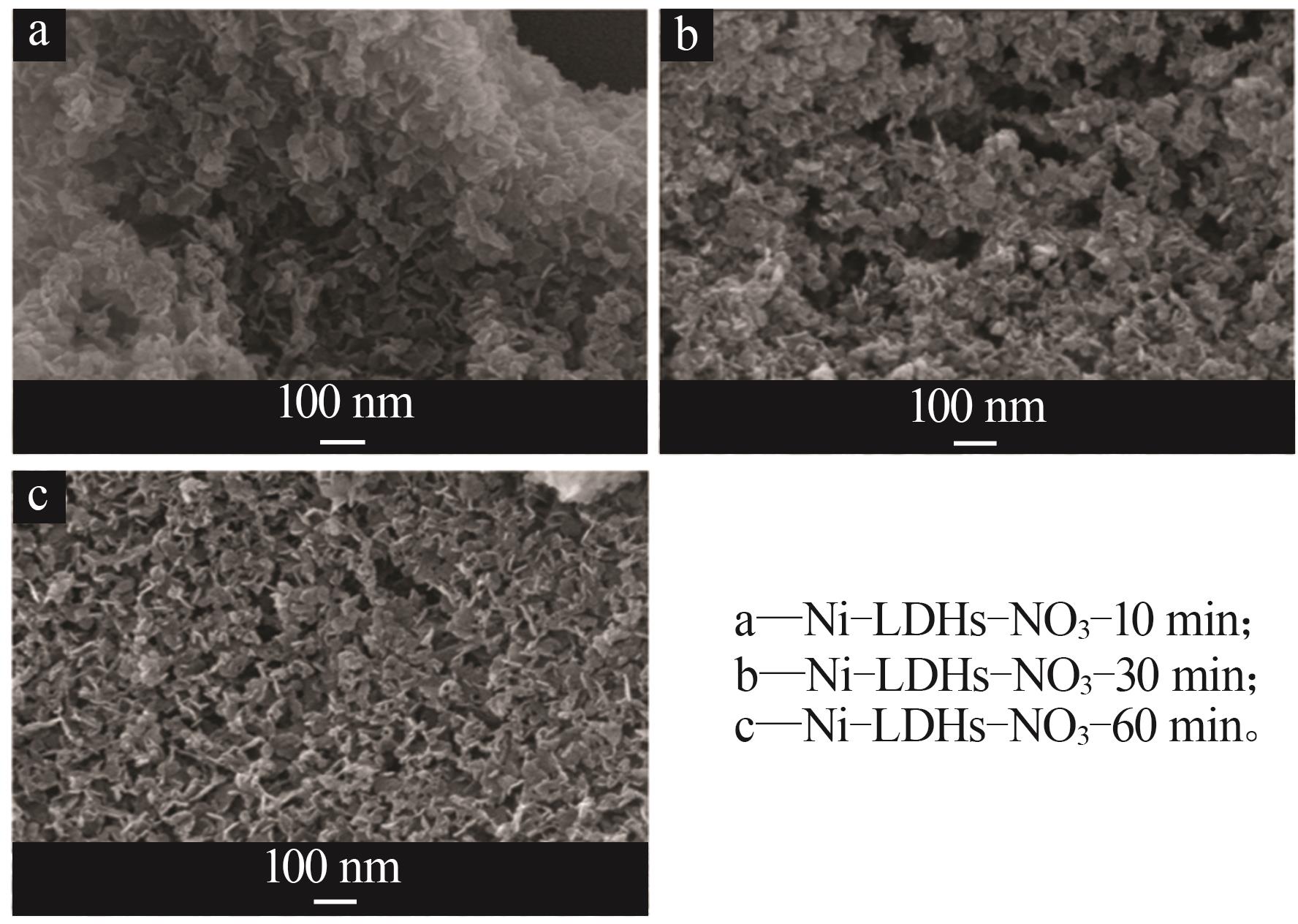
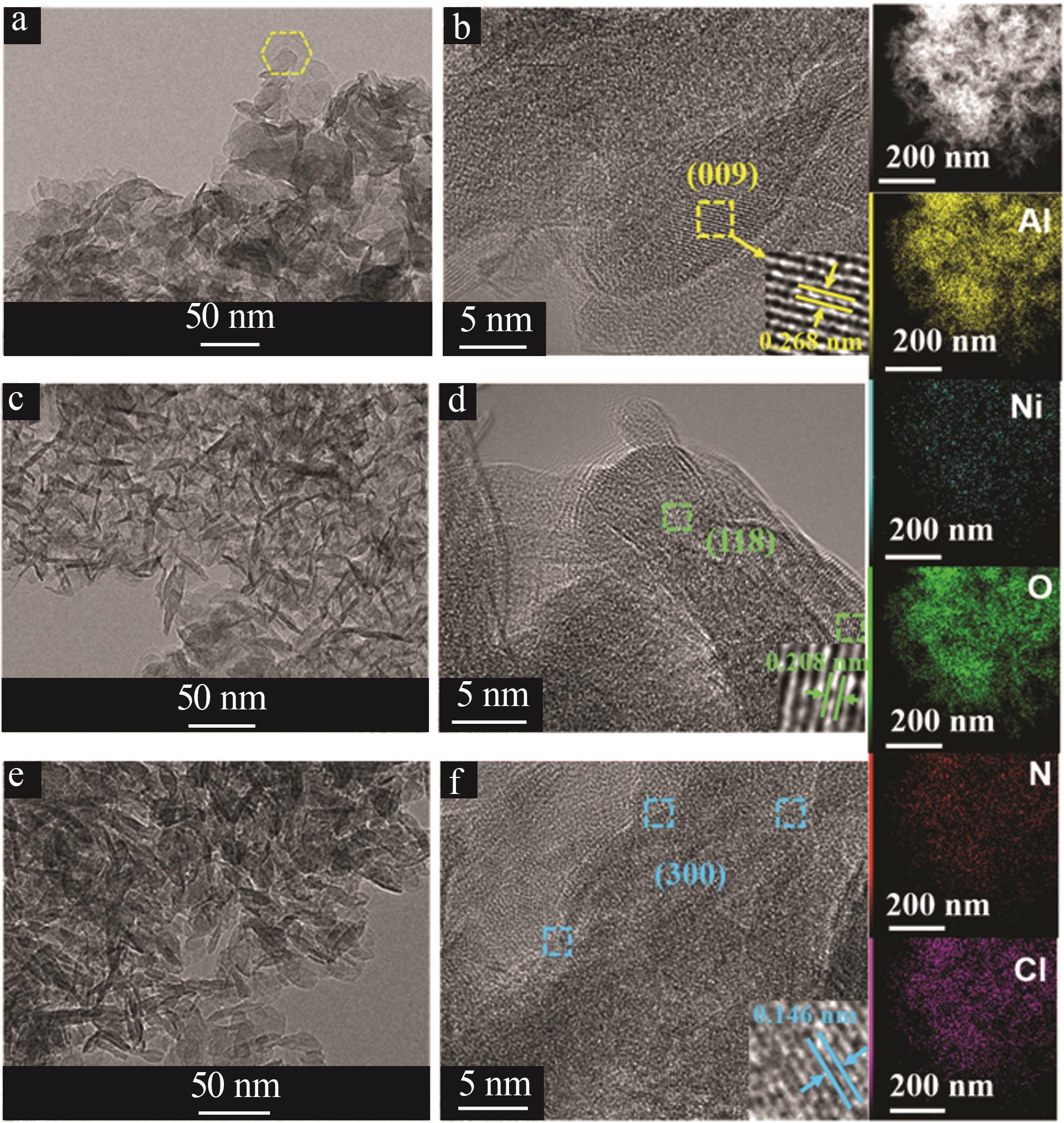
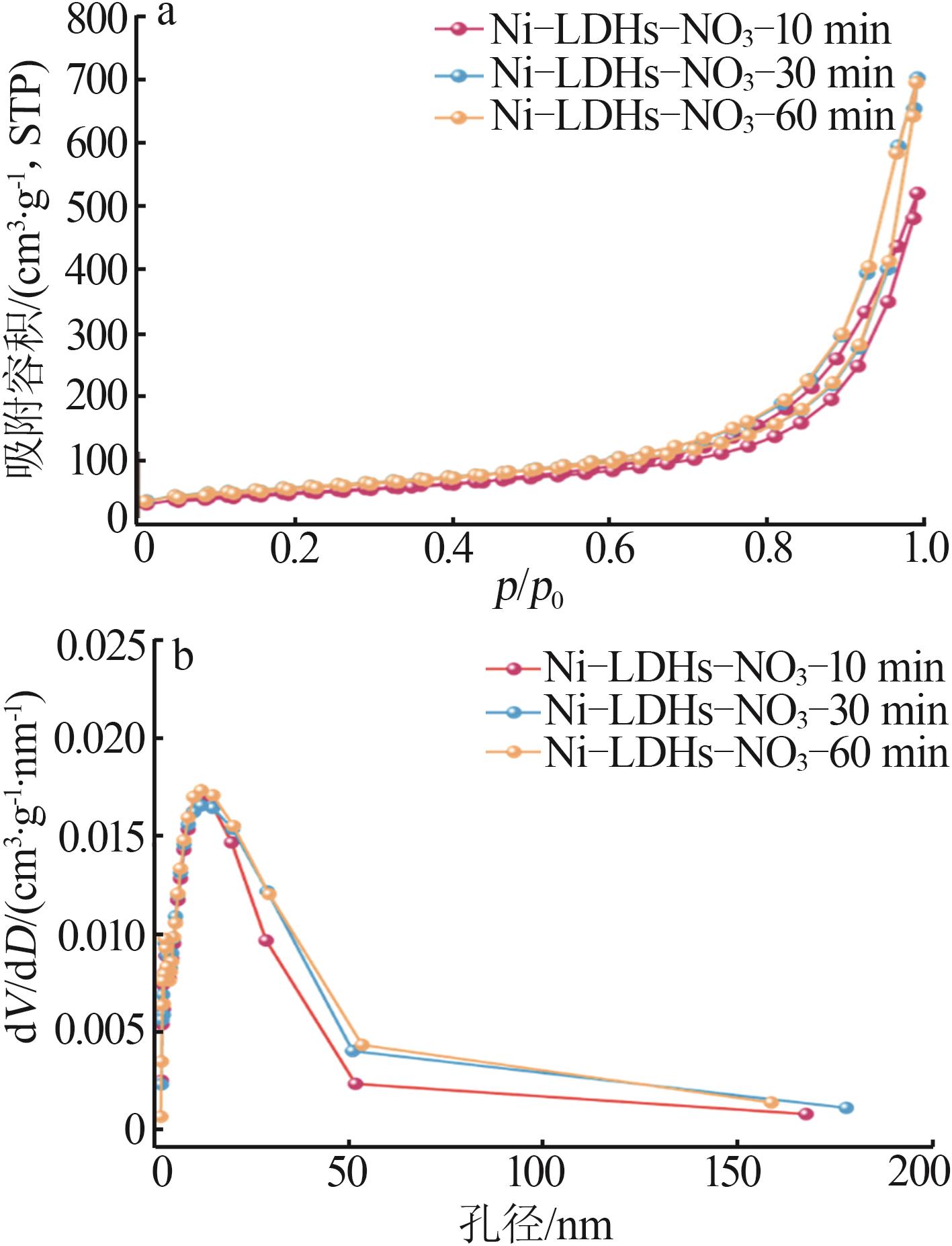
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (10): 32-40.doi: 10.19964/j.issn.1006-4990.2024-0516
• Research & Development • Previous Articles Next Articles
Study on nickel doping to construct structurally stable and high adsorption capacity aluminum-based adsorbents
LI Xuequn1,3( ), HUO Junjie2, LUO Wentian2, HE Wanghai1,3, SUN Hongbo1,3, YU Xudong2, HAI Chunxi2(
), HUO Junjie2, LUO Wentian2, HE Wanghai1,3, SUN Hongbo1,3, YU Xudong2, HAI Chunxi2( ), ZHOU Yuan2(
), ZHOU Yuan2( ), ZENG Ying2(
), ZENG Ying2( )
)
- 1. Qinghai CITIC Guoan Lithium Industry Development Co. ,Ltd. ,Golmud 816000,China
2. College of Materials and Chemistry and Chemical Engineering,Chengdu University of Technology,Chengdu 610059,China
3. Qinghai Key Laboratory of Comprehensive Utilization of Sulfate-type Salt Lake Resources,Xining 810008,China
-
Received:2024-09-29Online:2025-10-10Published:2025-01-15 -
Contact:HAI Chunxi, ZHOU Yuan, ZENG Ying E-mail:zzcl_yx@aliyun.com;haicx0628@163.com;yzhou712@sina.com;zengyster@163.com
CLC Number:
Cite this article
LI Xuequn, HUO Junjie, LUO Wentian, HE Wanghai, SUN Hongbo, YU Xudong, HAI Chunxi, ZHOU Yuan, ZENG Ying. Study on nickel doping to construct structurally stable and high adsorption capacity aluminum-based adsorbents[J]. Inorganic Chemicals Industry, 2025, 57(10): 32-40.
share this article
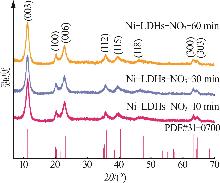
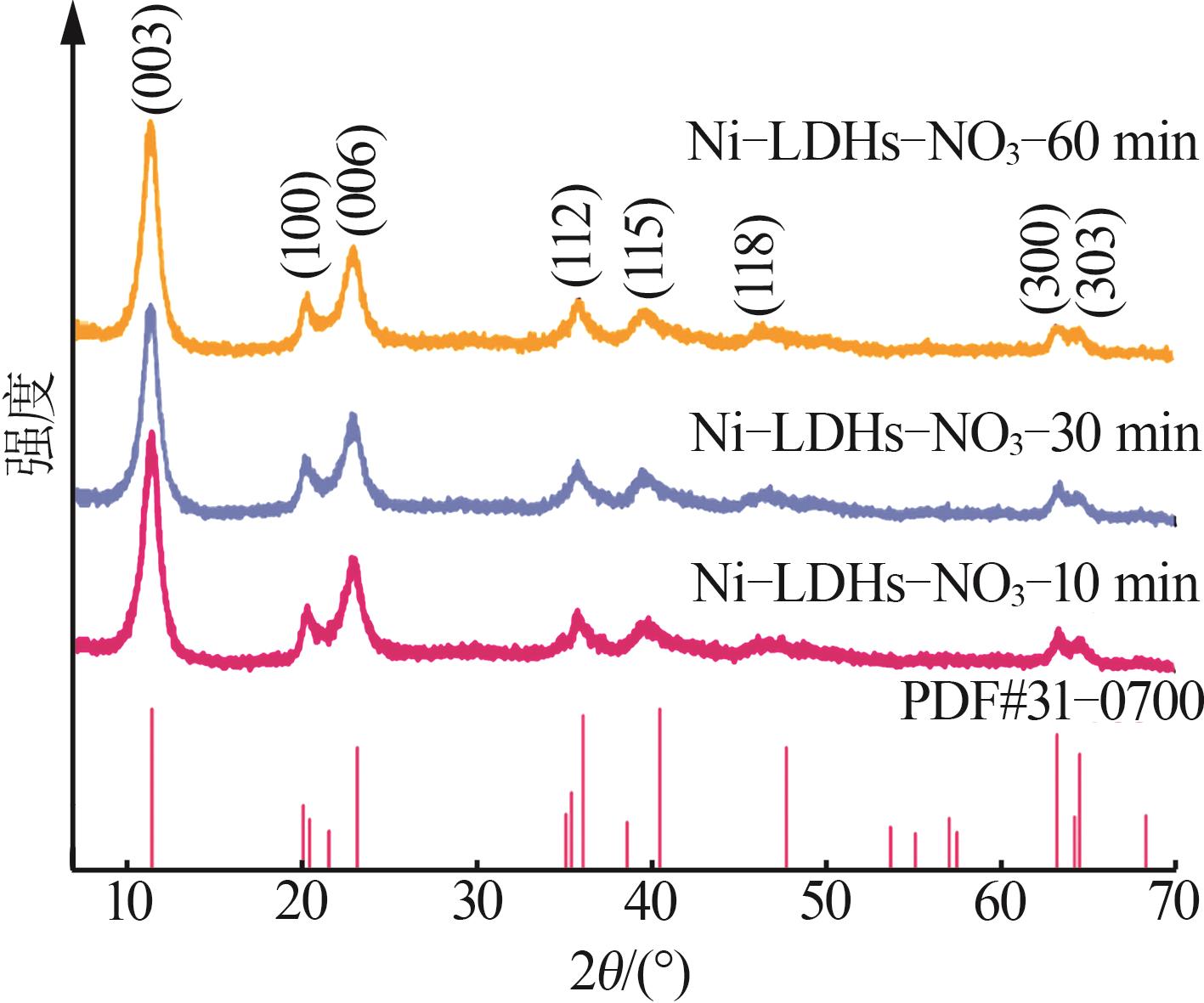
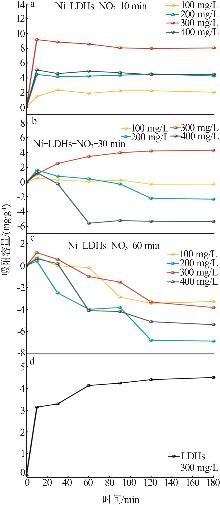
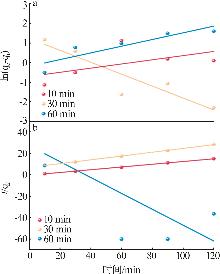
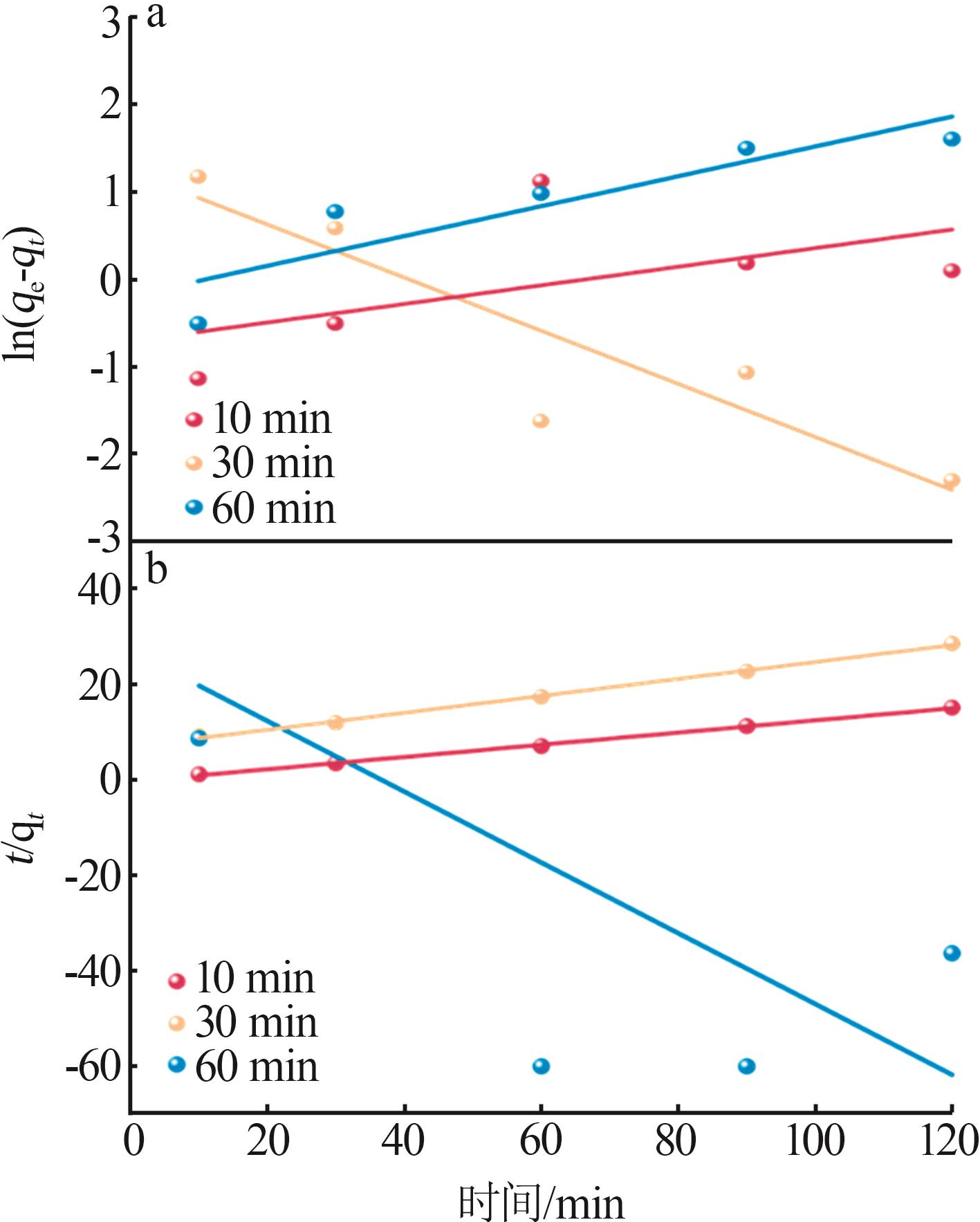
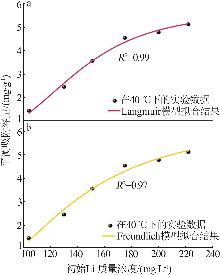
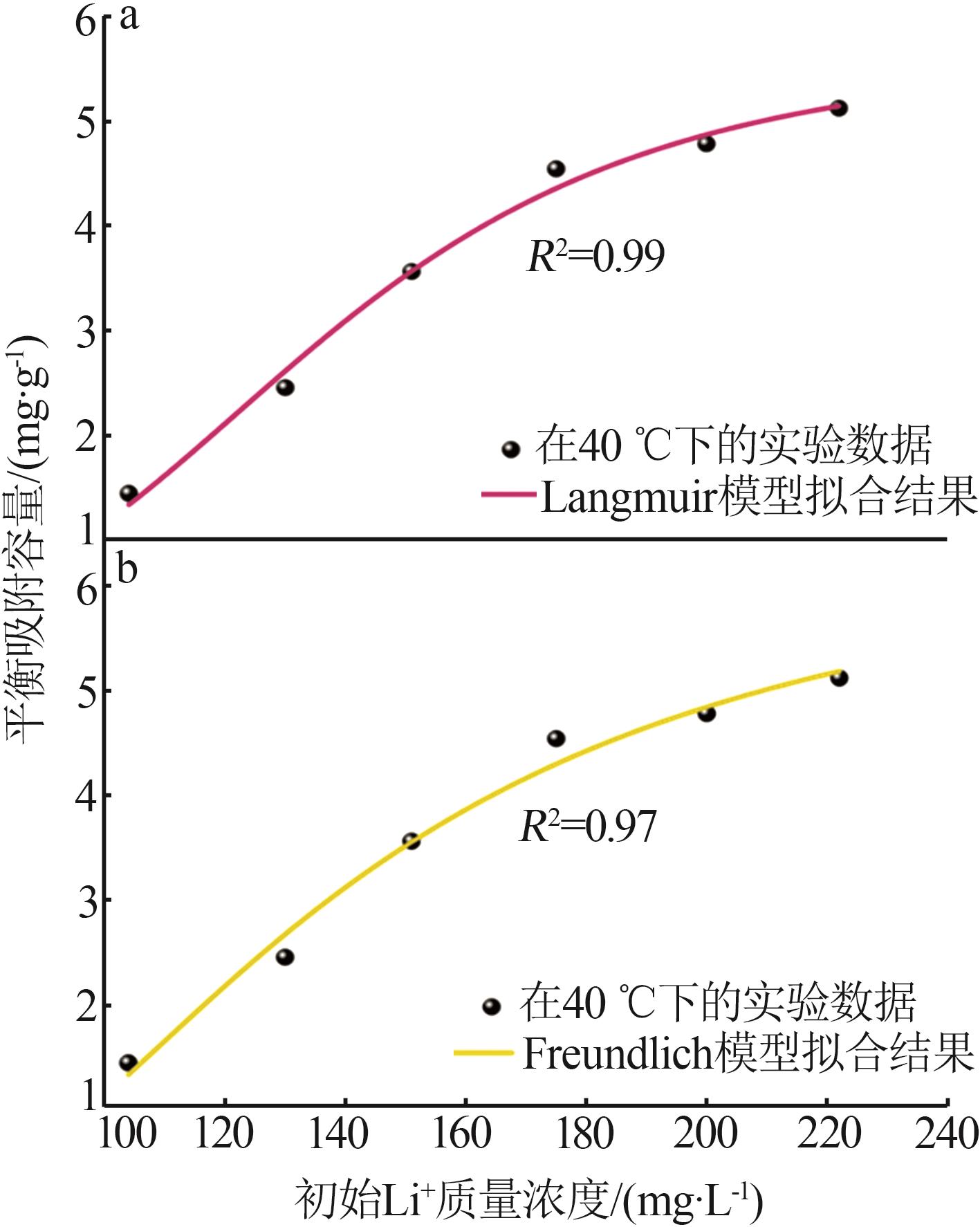
Table 2
Experimental value,conversion value and fitting parameters of pseudo-first-order and pseudo-second-order kinetics at 40 ℃"
| 吸附剂 | Qe/ (mg·g-1) | 准一级动力学 | 准二级动力学 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
qe1/ (mg·g-1) | k1/min-1 | R2 | 残差 平方和 | qe2/ (mg·g-1) | k2/ (g·mg-1·min-1) | R2 | 残差 平方和 | ||||
| Ni-LDHs-NO3-10 min | 9.18 | 0.491 | 0.010 | 0.085 | 1.947 | 7.833 | 0.042 | 0.998 | 0.136 | ||
| Ni-LDHs-NO3-30 min | 4.32 | 3.441 | 0.030 | 0.785 | 1.395 | 5.646 | 0.004 | 0.997 | 0.487 | ||
| Ni-LDHs-NO3-60 min | 1.16 | 0.824 | 0.017 | 0.742 | 0.550 | -1.35 | 0.020 | 0.260 | 5 376.993 | ||
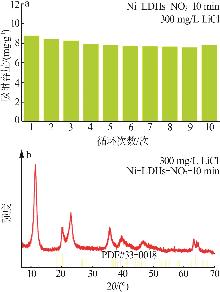
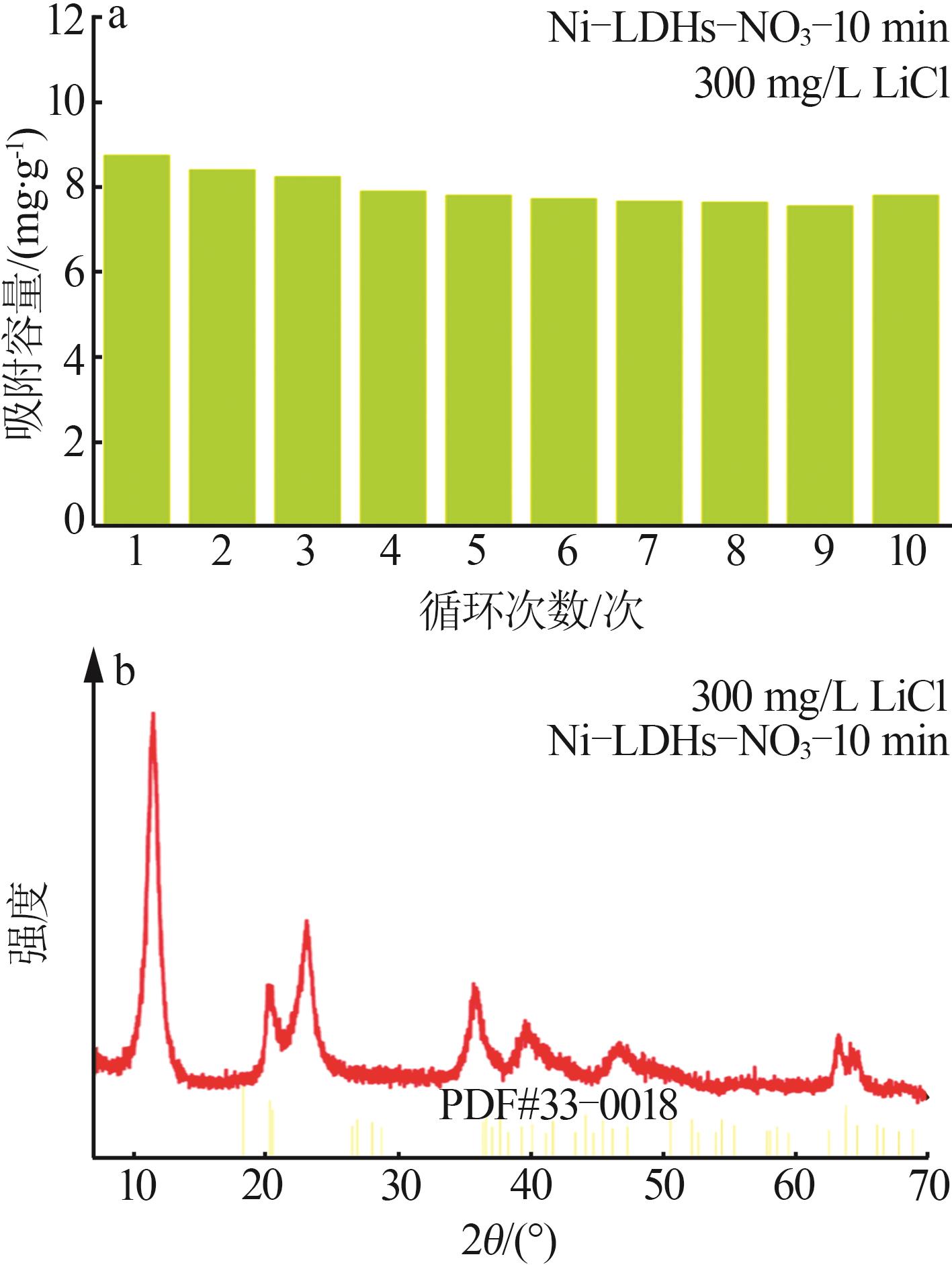
Table 4
Comparison of Li+ adsorption performance of different aluminum-based lithium adsorbents"
| 吸附剂 | 最大吸附容 量/(mg·g-1) | 吸附条件 |
|---|---|---|
| Li/Al-LDH[ | 7.12 | 察尔汗老卤,399 mg/L |
| MLDHs[ | 5.83 | 察尔汗老卤,399 mg/L |
| LiZnAl-LDH-5%[ | 7.00 | 罗布泊卤水,222.2 mg/L |
| Fe3O4@LiAl-LDH[ | 6.39 | 察尔汗老卤,399 mg/L |
| LiAl-LDH[ | 5.69 | 真实卤水,350 mg/L |
| Li-Al-Fe-Cl LDH[ | 11.30 | 模拟卤水,800 mg/L |
| Ni-LDHs-NO3-10 min | 9.18 | 模拟锂溶液,300 mg/L |
| [1] | DUNN B, KAMATH H, TARASCON J M.Electrical energy storage for the grid:A battery of choices[J].Science,2011,334(6058):928-935. |
| [2] | YANG Sixie, ZHANG Fan, DING Huaiping,et al.Lithium metal extraction from seawater[J].Joule,2018,2(9):1648-1651. |
| [3] | VERA M L, TORRES W R, GALLI C I,et al.Environmental impact of direct lithium extraction from brines[J].Nature Reviews Earth & Environment,2023,4(3):149-165. |
| [4] | DELAPORTE N, LAJOIE G, DARWICHE A,et al.Stabilization of lithium anode with ceramic-rich interlayer for all solid-state batteries[J].RSC Advances,2022,12(24):15493-15507. |
| [5] | 靳佳奇,李岩,林森.盐湖卤水吸附提锂技术研究进展[J].化学工程,2023,51(5):20-25. |
| JIN Jiaqi, LI Yan, LIN Sen.Research progress of lithium extraction from salt lake brine by adsorption[J].Chemical Engineering (China),2023,51(5):20-25. | |
| [6] | 罗清龙,董明哲,李军,等.吸附法分离盐湖卤水中锂的研究进展[J].盐湖研究,2023,31(1):106-115. |
| LUO Qinglong, DONG Mingzhe, LI Jun,et al.Research progress of lithium separation from salt lake brine by adsorption method[J].Journal of Salt Lake Research,2023,31(1):106-115. | |
| [7] | 陈仰,李欢,顾升波,等.盐湖锂资源现状及提锂技术研究进展[J].工程科学学报,2024,46(9):1659-1670. |
| CHEN Yang, LI Huan, GU Shengbo,et al.Present situation of salt-lake lithium resources and research progress of lithium extraction technology[J].Chinese Journal of Engineering,2024,46(9):1659- 1670. | |
| [8] | 乜贞,伍倩,丁涛,等.中国盐湖卤水提锂产业化技术研究进展[J].无机盐工业,2022,54(10):1-12. |
| NIE Zhen, WU Qian, DING Tao,et al.Research progress on industrialization technology of lithium extraction from salt lake brine in China[J].Inorganic Chemicals Industry,2022,54(10):1-12. | |
| [9] | 王琪,赵有璟,刘洋,等.高镁锂比盐湖镁锂分离与锂提取技术研究进展[J].化工学报,2021,72(6):2905-2921,3433. |
| WANG Qi, ZHAO Youjing, LIU Yang,et al.Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine with high magnesium/lithium ratio[J].CIESC Journal,2021,72(6):2905-2921,3433. | |
| [10] | WU Lei, ZHANG Changyong, KIM S,et al.Lithium recovery using electrochemical technologies:Advances and challenges[J].Water Research,2022,221:118822. |
| [11] | TABELIN C B, DALLAS J, CASANOVA S,et al.Towards a low-carbon society:A review of lithium resource availability,challenges and innovations in mining,extraction and recycling,and future perspectives[J].Minerals Engineering,2021,163:106743. |
| [12] | ZHAI Panlong, XIA Mingyue, WU Yunzhen,et al.Engineering single-atomic ruthenium catalytic sites on defective nickel-iron layered double hydroxide for overall water splitting[J].Nature Communications,2021,12:4587. |
| [13] | 许乃才,史丹丹,黎四霞,等.利用吸附技术提取盐湖卤水中锂的研究进展[J].材料导报,2017,31(17):116-121. |
| XU Naicai, SHI Dandan, LI Sixia,et al.Advances in extracting lithium from salt-lake brines by adsorption technique[J].Materials Review,2017,31(17):116-121. | |
| [14] | WEI Shudan, WEI Yuanfeng, CHEN Tao,et al.Porous lithium ion sieves nanofibers:General synthesis strategy and highly selective recovery of lithium from brine water[J].Chemical Engineering Journal,2020,379:122407. |
| [15] | WU Qian, BU Lingzhong, ZHANG Jintao,et al.Study of the optimization of the stereo-crystallization process with enhanced salinity-gradient solar pond for lithium extraction from Zabuye salt lake in Tibet[J].Carbonates and Evaporites,2024,39(2):10. |
| [16] | ZHONG Jing, LIN Sen, YU Jianguo.Li+ adsorption performance and mechanism using lithium/aluminum layered double hydroxides in low grade brines[J].Desalination,2021,505:114983. |
| [17] | ZHANG Liyuan, ZHOU Dali, YAO Qianqian,et al.Preparation of H2TiO3-lithium adsorbent by the sol-gel process and its adsorption performance[J].Applied Surface Science,2016,368:82-87. |
| [18] | ZHANG Qinhui, LI Shaopeng, SUN Shuying,et al.LiMn2O4 spinel direct synthesis and lithium ion selective adsorption[J].Chemical Engineering Science,2010,65(1):169-173. |
| [19] | CHEN Jun, LIN Sen, YU Jianguo.Quantitative effects of Fe3O4 nanoparticle content on Li+ adsorption and magnetic recovery performances of magnetic lithium-aluminum layered double hydroxides in ultrahigh Mg/Li ratio brines[J].Journal of Hazardous Materials,2020,388:122101. |
| [20] | PARANTHAMAN M P, LI Ling, LUO Jiaqi,et al.Recovery of lithium from geothermal brine with lithium-aluminum layered double hydroxide chloride sorbents[J].Environmental Science & Technology,2017,51(22):13481-13486. |
| [21] | SUN Ying, GUO Xiaoyu, HU Shaofang,et al.Highly efficient extraction of lithium from salt lake brine by LiAl-layered double hydroxides as lithium-ion-selective capturing material[J].Journal of Energy Chemistry,2019,34:80-87. |
| [22] | GRAHAM T R, HU Jianzhi, ZHANG Xin,et al.Unraveling gibbsite transformation pathways into LiAl-LDH in concentrated lithium hydroxide[J].Inorganic Chemistry,2019,58(18):12385-12394. |
| [23] | WANG Shanli, LIN C H, YAN Yayi,et al.Synthesis of Li/Al LDH using aluminum and LiOH[J].Applied Clay Science,2013,72:191-195. |
| [24] | ZHAO Kaiyu, TONG Bojia, YU Xiaoping,et al.Synthesis of porous fiber-supported lithium ion-sieve adsorbent for lithium recovery from geothermal water[J].Chemical Engineering Journal,2022,430:131423. |
| [25] | BAO Luri, ZHANG Jingze, TANG Weiping,et al.Synthesis and adsorption properties of metal oxide-coated lithium ion-sieve from salt lake brine[J].Desalination,2023,546:116196. |
| [26] | AL-DHAWI B N S, KUTTY S R M, BALOO L,et al.Lithium adsorption from aqueous solution using aluminum hydroxide:Characterization,optimization by response surface methodology,kinetic modelling,and isotherm studies[J].Case Studies in Chemical and Environmental Engineering,2023,7:100350. |
| [27] | JIANG Huixiong, YANG Ying, SUN Shuying,et al.Adsorption of lithium ions on lithium-aluminum hydroxides:Equilibrium and kinetics[J].The Canadian Journal of Chemical Engineering,2020,98(2):544-555. |
| [28] | AKBARI ZIARANI P, MOLAEI DEHKORDI A.Lithium extraction from an aqueous medium through in situ synthesis of aluminum hydroxide:A comprehensive study on adsorption and desorption processes,kinetics,isotherm models,and thermodynamic parameters[J].Industrial & Engineering Chemistry Research,2024,63(1):445-458. |
| [29] | ZHONG Jing, LIN Sen, YU Jianguo.Effects of excessive lithium deintercalation on Li+ adsorption performance and structural stability of lithium/aluminum layered double hydroxides[J].Journal of Colloid and Interface Science,2020,572:107-113. |
| [30] | CHEN Jun, DU Jianglong, YU Jianguo,et al.A one-step regeneration method in situ for deactivated aluminum-based lithium adsorbent used in high Mg2+/Li+ brines[J].Desalination,2023,554:116491. |
| [31] | ZHOU Haodong, LI Junfeng, XU Lei,et al.Efficient regeneration of the crystal structure and Li+ adsorption capacity of Li/Al layered double hydroxides[J].Materials Letters,2023,340:134159. |
| [32] | LV Shuaike, ZHAO Yunliang, ZHANG Lingjie,et al.Anion regulation strategy of lithium-aluminum layered double hydroxides for strengthening resistance to deactivation in lithium recovery from brines[J].Chemical Engineering Journal,2023,472:145026. |
| [33] | ZHANG Lingjie, ZHANG Tingting, ZHAO Yunliang,et al.Doping engineering of lithium-aluminum layered double hydroxides for high-efficiency lithium extraction from salt lake brines[J].Nano Research,2024,17(3):1646-1654. |
| [34] | ABBASI M, SABZEHMEIDANI M M, GHAEDI M,et al.Facile fabrication of leaf coral-like structured Cu-Al LDH/PVDF composite adsorptive membrane with enhanced adsorption performance[J].Materials Science and Engineering:B,2021,267:115086. |
| [35] | ABBASI M, SABZEHMEIDANI M M, GHAEDI M,et al.Synthesis of grass-like structured Mn-Fe layered double hydroxides/PES composite adsorptive membrane for removal of malachite green[J].Applied Clay Science,2021,203:105946. |
| [36] | SIMONIN J P.On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics[J].Chemical Engineering Journal,2016,300:254-263. |
| [37] | ZHANG Lingjie, ZHENG Siyin, LI Peng,et al.Resource utilization of organic spent adsorbent to prepare three-dimensional sulfate-functionalized layered double oxide for superior removal of azo dye[J].Environmental Science and Pollution Research,2021, 28(38):53021-53033. |
| [38] | CHEN Jun, LIN Sen, YU Jianguo.High-selective cyclic adsorption and magnetic recovery performance of magnetic lithium-aluminum layered double hydroxides(MLDHs) in extracting Li+ from ultrahigh Mg/Li ratio brines[J].Separation and Purification Technology,2021,255:117710. |
| [39] | WANG Dongdong, ZHU Qi, SU Yingying,et al.Preparation of MgAlFe-LDHs as a deicer corrosion inhibitor to reduce corrosion of chloride ions in deicing salts[J].Ecotoxicology and Environmental Safety,2019,174:164-174. |
| [40] | ZHANG Lingjie, KE Zhisheng, WANG Wenzhe,et al.Enhanced removal of multiple metal ions on S-doped graphene-like carbon-supported layered double oxide:Mechanism and DFT study[J].Separation and Purification Technology,2022,288:120636. |
| [41] | HOU Lei, XING Baolin, KANG Weiwei,et al.Aluminothermic reduction synthesis of porous silicon nanosheets from vermiculite as high-performance anode materials for lithium-ion batteries[J].Applied Clay Science,2022,218:106418. |
| [42] | 余关龙,彭海渊,王世涛,等.固定化生物吸附剂对Cd(Ⅱ)的去除性能及机理[J].化工进展,2021,40(5):2882-2892. |
| YU Guanlong, PENG Haiyuan, WANG Shitao,et al.Performance and mechanism of immobilized biological adsorbent for Cd(Ⅱ) removal[J].Chemical Industry and Engineering Progress,2021,40(5):2882-2892. | |
| [43] | ZHANG Guotai, Chunxi HAI, ZHOU Yuan,et al.Al and F ions co-modified Li1.6Mn1.6O4 with obviously enhanced Li+ adsorption performances[J].Chemical Engineering Journal,2022,450:137912. |
| [44] | MENG Zhixiang, WANG Meiling, CAO Xun,et al.Highly flexible interconnected Li+ ion-sieve porous hydrogels with self-regulating nanonetwork structure for marine lithium recovery[J].Chemical Engineering Journal,2022,445:136780. |
| [45] | ZHOU Fang, HUANG Suhua, LIU Xu,et al.Adsorption kinetics and thermodynamics of rare earth on Montmorillonite modified by sulfuric acid[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2021,627:127063. |
| [46] | JIANG Huixiong, ZHANG Shuiyi, YANG Ying,et al.Synergic and competitive adsorption of Li-Na-MgCl2 onto lithium-aluminum hydroxides[J].Adsorption,2020,26(7):1039-1049. |
| [47] | LI Yuanyuan, TANG Na, ZHANG Lei,et al.Fabrication of Fe-doped lithium-aluminum-layered hydroxide chloride with enhanced reusable stability inspired by computational theory and its application in lithium extraction[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2023,658:130641. |
| [1] | WAN Feng, YAN Yingchun, FAN Zhuangjun. Research progress and prospect of halide solid electrolytes [J]. Inorganic Chemicals Industry, 2024, 56(11): 15-29. |
| [2] | YUAN Changmei,GUO Jun,ZHANG Dan,ZHANG Taiwen. Synthesis,structure and catalytic oxidation performance of iodine ion of Cu-3-trz-PMo12 [J]. Inorganic Chemicals Industry, 2022, 54(8): 145-150. |
| [3] | MAO Xinyu,WANG Yubin,WANG Wenwen,LI Shuqin,MA Xiaoxiao. Effect of alternating external magnetic field on the dissolution behavior of calcium sulfate scale and its mechanism [J]. Inorganic Chemicals Industry, 2022, 54(3): 97-101. |
| [4] | Feng Xiao,Guo Jun,Zhang Dan. Study on synthesis,structure and catalytic iodide oxidation of (C5H7N2O2)4(SiMo12O40) [J]. Inorganic Chemicals Industry, 2021, 53(9): 109-113. |
| [5] | Liu Yuanhui,Tang Xiaona,Xie Lei,Jiang Xiaopeng,Zhang Yunbo. Investigation progress of ageing process of plaster of paris [J]. Inorganic Chemicals Industry, 2020, 52(9): 15-20. |
| [6] | Wang Qianqian,Bai Chunhua,Ren Hui,Li Guanghui. Crystal structure regulation of calcium carbonate by adding tetradecanoic acid [J]. Inorganic Chemicals Industry, 2020, 52(4): 29-32. |
| [7] | Lü Pin,Shi Chunhui,Li Wei,Zhong Jianchu. Controllable synthesis and characterization of thermal stabilizer Mg-Al-LDHs [J]. Inorganic Chemicals Industry, 2019, 51(6): 29-33. |
| [8] | DI Jun, HUANG Chun-Hui, ZHANG Qin, ZHAO Na, ZONG Jun. Research on preparation of heavy MgO by brucite-alkali method [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 33-. |
| [9] | WANG Tao, MENG Qing-Xiang, XU Hai-Tao, XU Zhen-Liang. Preparation and characterization of lithium ion sieve nanofibrous adsorbents [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(3): 29-. |
| [10] | . Study on synthesis of easing soluble aluminum hydroxide by carbonation decomposition [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(6): 47-. |
| [11] | JIAO Yun-Hong, YU Shuang-Hong, SHI Ling, XU Jian-Zhong, GUO Bing-Ru. Preparation of lanthanum stannate by precipitation-calcination method [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(5): 27-. |
| [12] | CAO Jian-Fang, HUANG Zi-Ping, SUN Chun-Yan, CHENG Chun-Chun. Hydrothermal synthesis and characterization of supramolecular compound [(4,4′-bipy)3H5][PMo10V2O40]·3H2O [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(9): 72-. |
| [13] | LIU Na, MA Ya-Lu, LI Xiao-Ceng, LI Guo-Shu, FANG Wu-Cheng. Research progress in preparation and intercalation performance of layered sodium silicate [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(4): 9-. |
| [14] | LI Jing, YANG Xiao-Jun, XU Wang-Sheng. Preparation of perovskite-type nano-sized lead titanate [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(6): 40-. |
| [15] | Zhang Jing;Zhang Yaping;Yu Lianqing;Zhong Xiaoliang. Preparation of nanotitania by hydrothermal method and progress in research of photocatalytic properties thereof [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(9): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||