Inorganic Chemicals Industry ›› 2022, Vol. 54 ›› Issue (8): 145-150.doi: 10.19964/j.issn.1006-4990.2021-0703
• Catalytic Materials • Previous Articles
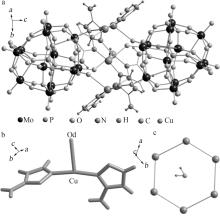
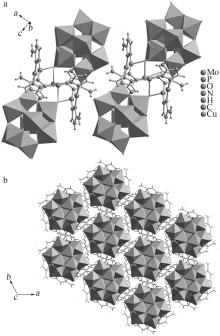
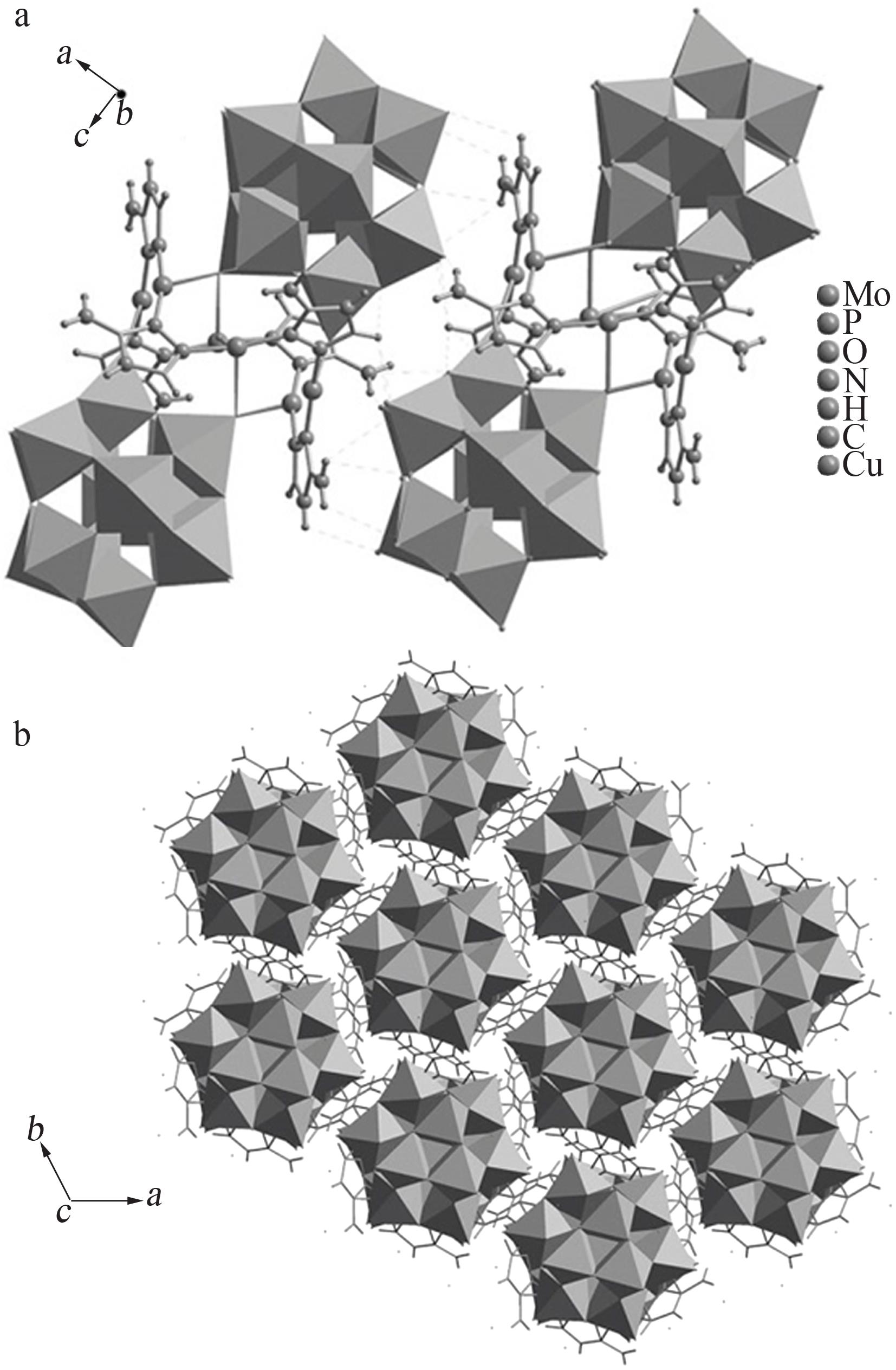
Synthesis,structure and catalytic oxidation performance of iodine ion of Cu-3-trz-PMo12
YUAN Changmei1( ),GUO Jun1,2(
),GUO Jun1,2( ),ZHANG Dan2,ZHANG Taiwen1
),ZHANG Dan2,ZHANG Taiwen1
- 1.School of Chemistry and Materials Science,Guizhou Normal University,Guiyang 550001,China
2.State Key Laboratory for Efficient Utilization of Medium and Low Grade Phosphate Rock and Its Associated Resources,Wengfu(Group) Co.
-
Received:2021-11-19Online:2022-08-10Published:2022-08-11 -
Contact:GUO Jun E-mail:522626103@qq.com;justin_gixt@163.com
CLC Number:
Cite this article
YUAN Changmei,GUO Jun,ZHANG Dan,ZHANG Taiwen. Synthesis,structure and catalytic oxidation performance of iodine ion of Cu-3-trz-PMo12[J]. Inorganic Chemicals Industry, 2022, 54(8): 145-150.
share this article
Table 1
Crystallographic data of compound"
晶体学 参数 | 分子式 | 相对分 子质量 | 晶体尺 寸/mm3 | 晶系 | 空间群 | a/nm | b/nm | c/nm | α/(°) | β/(°) |
|---|---|---|---|---|---|---|---|---|---|---|
| 数据结果 | 2(C6H12Cu3Mo12N12O40P)·H2O | 4 548.31 | 0.11×0.1× 0.08 | 三方 | R-3 | 1.756 66(3) | 1.756 66(3) | 2.303 24(6) | 90 | 90 |
晶体学 参数 | γ/(°) | V/nm 3 | Z | μ/mm-1 | F(000) | θmin, θmax/(°) | 波长/ nm | Dx/ (g?cm-3) | R1,wR2 [I>=2σ(I)] | R1,wR2 [all data] |
| 数据结果 | 120 | 6.155 2(3) | 3 | 5.223 | 6 378.0 | 2.218~ 24.988 | 0.071 073 | 3.681 | 0.023 8, 0.058 7 | 0.025 0,0.059 2 |
Table 2
Partial hydrogen bond parameters for the compound"
| D-H···A | d(D—H)/ nm | d(H—A)/ nm | d(D···A)/ nm | ∠(D—H···A)/(°) |
|---|---|---|---|---|
| N(4)—H(4B)···O(1) | 0.088 0 | 0.273 7 | 0.320 9 | 115.0 |
| N(3)—H(3)···O(4) | 0.088 0 | 0.273 7 | 0.312 8 | 108.3 |
| N(4)—H(4A)···O(7) | 0.088 0 | 0.301 8 | 0.337 0 | 106.1 |
| C(1)—H(1)···O(9) | 0.095 1 | 0.273 5 | 0.355 3 | 144.7 |
| C(1)—H(1)···O(7) | 0.095 1 | 0.281 4 | 0.335 5 | 117.1 |
| 1 | 宋锡高.中国碘素产业发展现状[J].无机盐工业,2014,46(3):9-12. |
| SONG Xigao.Present development situation of China′s iodine industry[J].Inorganic Chemicals Industry,2014,46(3):9-12. | |
| 2 | 张红映,雷学联.中国碘资源和碘化工生产与消费[J].磷肥与复肥,2011,26(2):76-78. |
| ZHANG Hongying, LEI Xuelian.Production and consumption of iodine resources and iodine chemical industry in China[J].Phosphate & Compound Fertilizer,2011,26(2):76-78. | |
| 3 | SCHREIBER M, VIVEKANANDHAN S, MOHANTY A K,et al.Iodine treatment of lignin-cellulose acetate electrospun fibers:Enhancement of green fiber carbonization[J].ACS Sustainable Chemistry & Engineering,2015,3(1):33-41. |
| 4 |
MADHU S, EVANS H A, DOAN-NGUYEN V V T,et al.Inside back cover:Infinite polyiodide chains in the pyrroloperylene⁃iodine complex:Insights into the starch⁃iodine and perylene⁃iodine complexes[J].Angewandte Chemie International Edition,2016,55(28).Doi:10.1002/anie.201604535 .
doi: 10.1002/anie.201604535 |
| 5 | ZHAO Qing, LU Yanying, ZHU Zhiqiang,et al.Rechargeable lithi⁃ |
| um⁃iodine batteries with iodine/nanoporous carbon cathode[J].Nano Letters,2015,15(9):5982-5987. | |
| 6 | ZHANG Yongna, Hongying LÜ, WANG Lu,et al.The oxidation of benzothiophene using the keggin⁃type lacunary polytungstophosphate as catalysts in emulsion[J].Journal of Molecular Catalysis A:Chemical,2010,332(1/2):59-64. |
| 7 | REZVANI M A, OVEISI M, ASLI M A NIA.Phosphotungestovanadate immobilized on PVA as an efficient and reusable nano catalyst for oxidative desulphurization of gasoline[J].Journal of Molecular Catalysis A:Chemical,2015,410:121-132. |
| 8 | BAI Xueli, HUANG Xin, WEN Liang,et al.A new strategy for the selective oxidation of alcohols catalyzed by a polyoxometalate⁃based hybrid surfactant in biphasic systems[J].Chemical Communications(Cambridge,England),2019,55(25):3598-3601. |
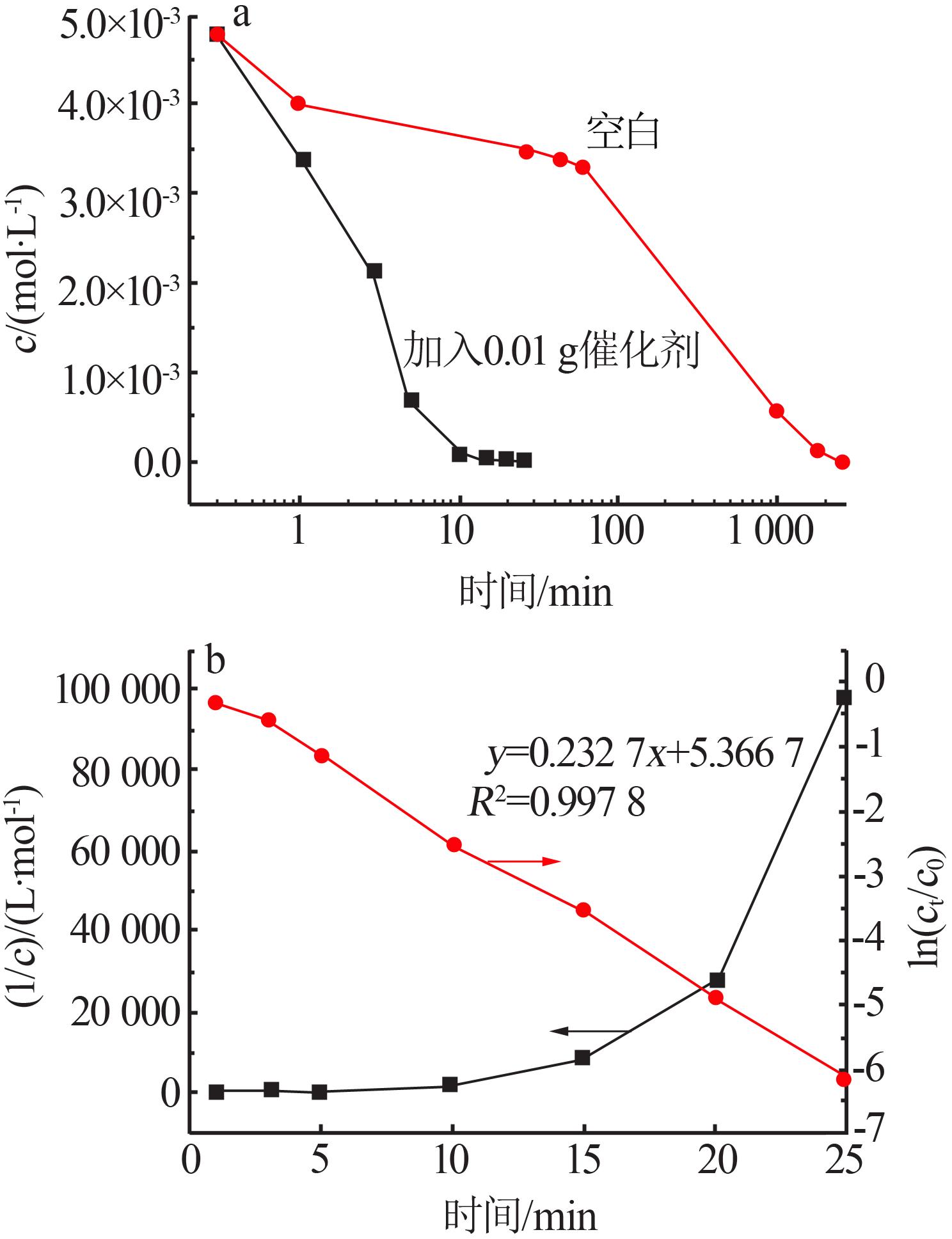
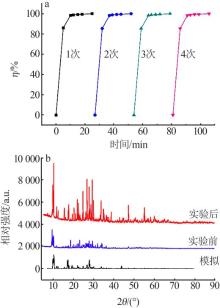
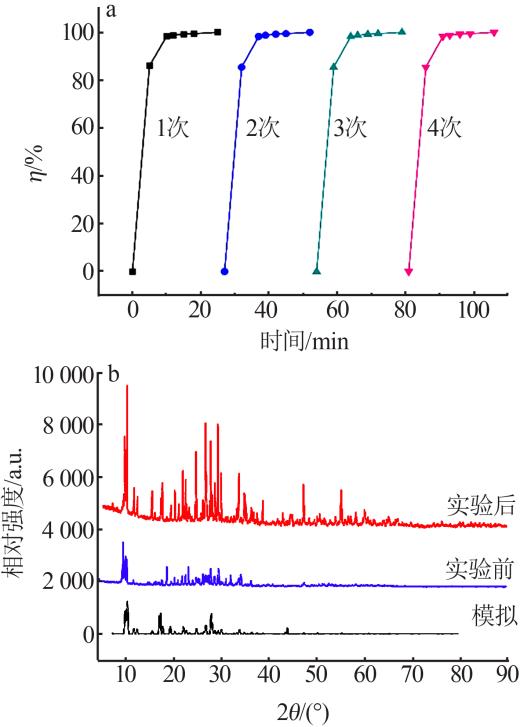
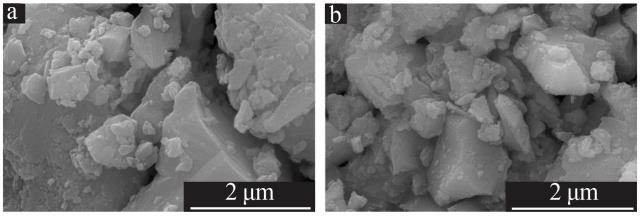
| 9 | 邬红龙,李文飞,郭军,等.碘吸收液中碘离子催化氧化结晶研究[J].广州化工,2014,42(15):54-57. |
| WU Honglong, LI Wenfei, GUO Jun,et al.Study on catalytic oxidation and crystallization of iodine ion in absorption liquid[J].Gu⁃ | |
| angzhou Chemical Industry,2014,42(15):54-57. | |
| 10 | 周维珍,郭军,李文飞,等.膨润土负载磷钼酸催化碘离子氧化反应的研究[J].无机盐工业,2017,49(10):62-66. |
| ZHOU Weizhen, GUO Jun, LI Wenfei,et al.Research on catalytic oxidation reaction of iodine ions by bentonite⁃phosphomo⁃ | |
| lybdic acid catalysts[J].Inorganic Chemicals Industry,2017,49(10):62-66. | |
| 11 | ZHU Yunfeng, ZHU Mingyuan, KANG Lihua,et al.Phosphotungstic acid supported on mesoporous graphitic carbon nitride as catalyst for oxidative desulfurization of fuel[J].Industrial & Engineering Chemistry Research,2015,54(7):2040-2047. |
| 12 | LIU Lingling, GUO Fangyuan, XU Jian,et al.Adsorption⁃enhanced oxidative desulfurization by a task⁃specific pyridinium⁃based porous ionic polymer[J].Fuel,2019,244:439-446. |
| 13 | REZVANI M A, MIRI O F.Synthesis and characterization of PWMn/NiO/PAN nanosphere composite with superior catalytic activity for oxidative desulfurization of real fuel[J].Chemical Engineering Journal,2019,369:775-783. |
| 14 | LI Na, MU Bao, LV Lei,et al.Assembly of new polyoxometalate-templated metal⁃organic frameworks based on flexible ligands[J].Journal of Solid State Chemistry,2015,226:88-93. |
| 15 | 赵婕,郭晴晴,郑玉国,等.2,6-二甲基-3,5-二(吡唑-3-基)吡啶/铜构筑的一维链状Keggin型杂多酸盐的合成、结构及电化学性质研究[J].人工晶体学报,2020,49(3):500-504,510. |
| ZHAO Jie, GUO Qingqing, ZHENG Yuguo,et al.Synthesis,structure and electrochemical properties of a 1D chain⁃like keggin⁃based hybrid constructed by 2,6-dimethyl-3,5-bis(pyrazole-3-yl) pyridine and copper[J].Journal of Synthetic Crystals,2020,49(3):500-504,510. | |
| 16 | LIU Dongsheng, CHEN Wentong, YE Guangming,et al.Synthesis and characterization of an inorganic-organic hybrid copper coordination polymer based on well⁃defined Keggin polyanions[J].Inorganica Chimica Acta,2018,477:84-88. |
| 17 | SHA Jingquan, PENG Jun, TIAN Aixiang,et al.Assembly of multitrack Cu-N coordination polymeric chain⁃modified polyoxome⁃ |
| talates influenced by polyoxoanion cluster and ligand[J].Crystal Growth & Design,2007,7(12):2535-2541. | |
| 18 | SHA Jingquan, PENG Jun, LIU Hongsheng,et al.Asymmetrical polar modification of a bivanadium-capped Keggin POM by multiple Cu-N coordination polymeric chains[J].Inorganic Chemistry,2007,46(26):11183-11189. |
| 19 | BROWN I D, ALTERMATT D.Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database[J].Acta Crystallographica Section B Structural Science,1985,41(4):244-247. |
| 上接第 124 页) | |
| TANG Mengqi, LI Xiangrong, LIU Guowen,et al.Determination of free α-SiO2 content in mill scale by X-ray diffraction K value method[J].Rock and Mineral Analysis,2015,34(5):565-569. | |
| 20 | 刘子铭,熊锐,关博文,等.不同pH值条件下水泥砂浆硫酸盐侵蚀损伤评价[J].硅酸盐通报,2016,35(7):2247-2253. |
| LIU Ziming, XIONG Rui, GUAN Bowen,et al.Damage evaluation of sulfate erosion to cement mortar under different pH-value conditions[J].Bulletin of the Chinese Ceramic Society,2016,35(7):2247-2253. | |
| 21 | 李涛,朱鹏涛,张彬,等.硫酸盐侵蚀下混凝土内腐蚀反应-扩散过程的实验研究[J].硅酸盐通报,2020,39(1):50-55. |
| LI Tao, ZHU Pengtao, ZHANG Bin,et al.Experimental study on corrosion reaction⁃diffusion process of concrete under sulfate attack[J].Bulletin of the Chinese Ceramic Society,2020,39(1):50-55. | |
| 22 | 高小建,马保国,邓红卫.胶凝材料组成对混凝土TSA硫酸盐侵蚀的影响[J].哈尔滨工业大学学报,2007,39(10):1554-1558. |
| GAO Xiaojian, MA Baoguo, DENG Hongwei.Influence of binder composition on the thaumasite form of sulfate attack of concre⁃ | |
| te[J].Journal of Harbin Institute of Technology,2007,39(10):1554-1558. |
| [1] | WAN Feng, YAN Yingchun, FAN Zhuangjun. Research progress and prospect of halide solid electrolytes [J]. Inorganic Chemicals Industry, 2024, 56(11): 15-29. |
| [2] | YUAN Enxian, LI Jinpeng, LI Qian, ZHOU Meixia, JIAN Panming. Preliminary study on cyclohexane catalytic oxidation over magnesium-doped tricobalt tetraoxide [J]. Inorganic Chemicals Industry, 2023, 55(6): 136-141. |
| [3] | GUO Jun, ZHANG Taiwen, ZHANG Dan. Synthesis,characterization of (PMo12)(HPy)3 and its catalytic oxidation property of iodide ion [J]. Inorganic Chemicals Industry, 2023, 55(4): 125-132. |
| [4] | MAO Xinyu,WANG Yubin,WANG Wenwen,LI Shuqin,MA Xiaoxiao. Effect of alternating external magnetic field on the dissolution behavior of calcium sulfate scale and its mechanism [J]. Inorganic Chemicals Industry, 2022, 54(3): 97-101. |
| [5] | Feng Xiao,Guo Jun,Zhang Dan. Study on synthesis,structure and catalytic iodide oxidation of (C5H7N2O2)4(SiMo12O40) [J]. Inorganic Chemicals Industry, 2021, 53(9): 109-113. |
| [6] | HAN Xinyu,LIU Kaijie,BIAN Mengyao,ZHANG Yibo,YANG Xiangguang. Catalytic performance study of Ce-MnOx for low-temperature purification of nitrogen oxides and carbon monoxide [J]. Inorganic Chemicals Industry, 2021, 53(12): 35-42. |
| [7] | Liu Yuanhui,Tang Xiaona,Xie Lei,Jiang Xiaopeng,Zhang Yunbo. Investigation progress of ageing process of plaster of paris [J]. Inorganic Chemicals Industry, 2020, 52(9): 15-20. |
| [8] | Wang Qianqian,Bai Chunhua,Ren Hui,Li Guanghui. Crystal structure regulation of calcium carbonate by adding tetradecanoic acid [J]. Inorganic Chemicals Industry, 2020, 52(4): 29-32. |
| [9] | Hu Min,Guo Jia,Wu Huadong,Zhang Linfeng. Photocatalytic oxidation of sodium sulfite in desulfurization wastewater by N-Zn/TiO2 [J]. Inorganic Chemicals Industry, 2020, 52(10): 151-156. |
| [10] | Lü Pin,Shi Chunhui,Li Wei,Zhong Jianchu. Controllable synthesis and characterization of thermal stabilizer Mg-Al-LDHs [J]. Inorganic Chemicals Industry, 2019, 51(6): 29-33. |
| [11] | DI Jun, HUANG Chun-Hui, ZHANG Qin, ZHAO Na, ZONG Jun. Research on preparation of heavy MgO by brucite-alkali method [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(9): 33-. |
| [12] | HUANG Li-Na, HOU Guang-Sheng, LI Bao-Shan. Study on oxidation of iodine ion in nature brine [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(1): 28-. |
| [13] | . Study on synthesis of easing soluble aluminum hydroxide by carbonation decomposition [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(6): 47-. |
| [14] | JIAO Yun-Hong, YU Shuang-Hong, SHI Ling, XU Jian-Zhong, GUO Bing-Ru. Preparation of lanthanum stannate by precipitation-calcination method [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(5): 27-. |
| [15] | CAO Jian-Fang, HUANG Zi-Ping, SUN Chun-Yan, CHENG Chun-Chun. Hydrothermal synthesis and characterization of supramolecular compound [(4,4′-bipy)3H5][PMo10V2O40]·3H2O [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(9): 72-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||