Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (7): 1-10.doi: 10.19964/j.issn.1006-4990.2023-0600
• Reviews and Special Topics • Next Articles
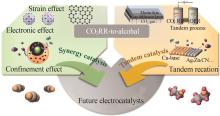
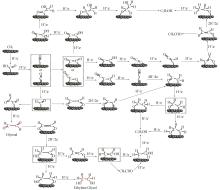
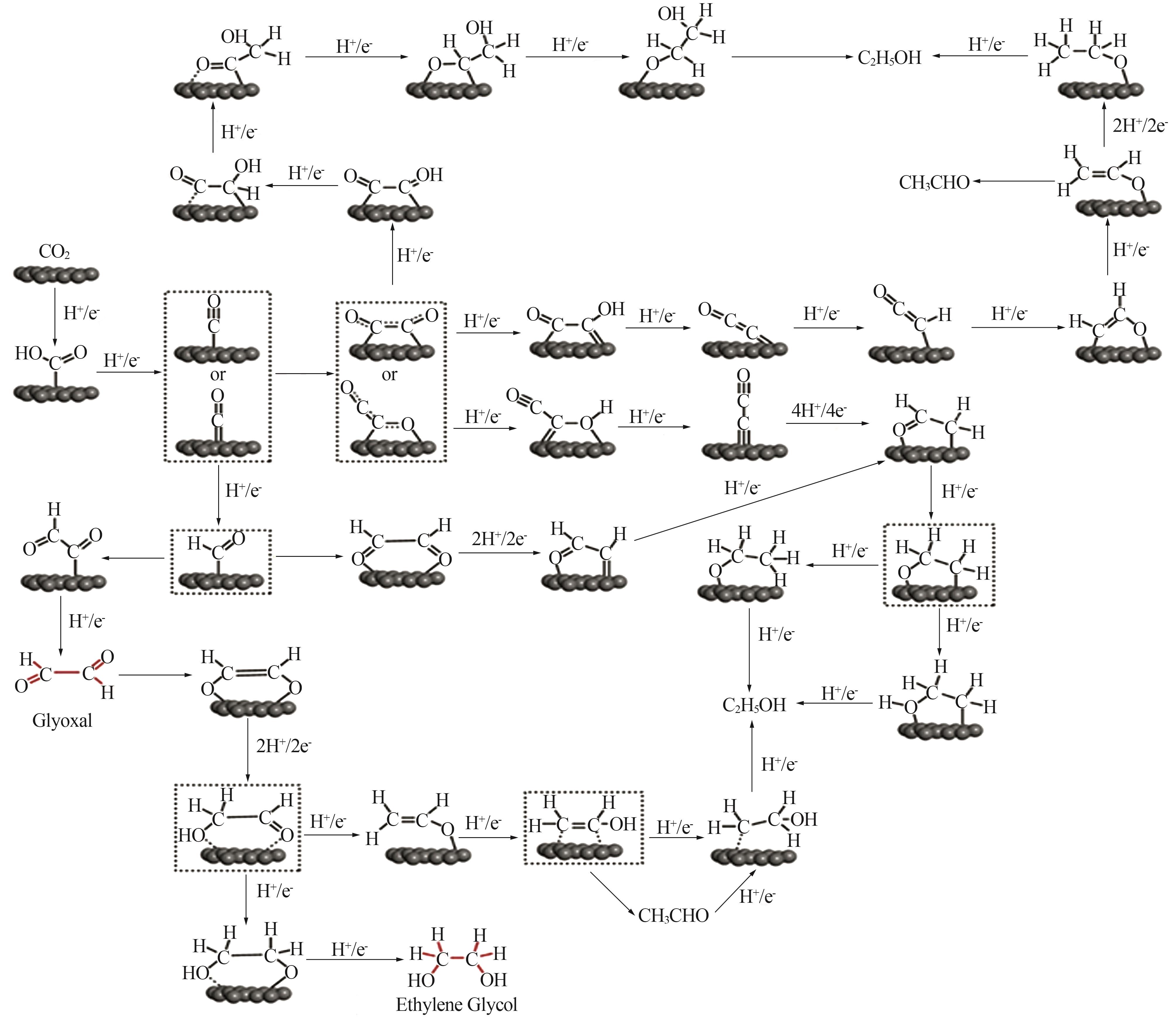
Research progress of catalytic system and materials for electrocatalytic reduction of carbon dioxide to ethanol
WANG Ting1( ), ZHANG Wenwen1, MAO Qing2(
), ZHANG Wenwen1, MAO Qing2( ), LÜ Li3, LIU Changzhen
), LÜ Li3, LIU Changzhen
- 1.China Nuclear Industry Zhongyuan Construction Co. ,Ltd. ,Beijing 100070,China
2.School of Chemical Engineering,Dalian University of Technology,Dalian 116023,China
3.State Key Laboratory of NBC Protection for Civilian,Beijing 100091,China
-
Received:2023-12-13Online:2024-07-10Published:2024-08-01 -
Contact:MAO Qing E-mail:twang800410@126.com;maoqing@dlut.edu.cn
CLC Number:
Cite this article
WANG Ting, ZHANG Wenwen, MAO Qing, LÜ Li, LIU Changzhen. Research progress of catalytic system and materials for electrocatalytic reduction of carbon dioxide to ethanol[J]. Inorganic Chemicals Industry, 2024, 56(7): 1-10.
share this article
Table 1
Comparison of different electrocatalysts performance"
| 电催化剂 | 过电位/ (V vs.RHE) | 乙醇法拉第 效率/% | 电流密度/ (mA∙cm-2) |
|---|---|---|---|
| Cu(Ag-20)20-M[ | -1.10 | 14.1 | 2.70 |
| OD-Ag15Cu85[ | -1.00 | 33.7 | 8.67 |
| Au/Cu[ | -0.90 | — | — |
| AuCu/Cu-SCA[ | -1.00 | 29±4 | — |
| Cu4Zn[ | -1.05 | 29.1 | 8.20 |
| Ag-G-NCF[ | -0.6~-0.7 | 82.0~85.2 | — |
| BND[ | -1.00 | 93.2 | — |
| RuPC/NPC[ | -0.87~-1.07 | 21.0~27.5 | — |
| MC-CNT/Co[ | -0.32 | 60.1 | 5.10 |
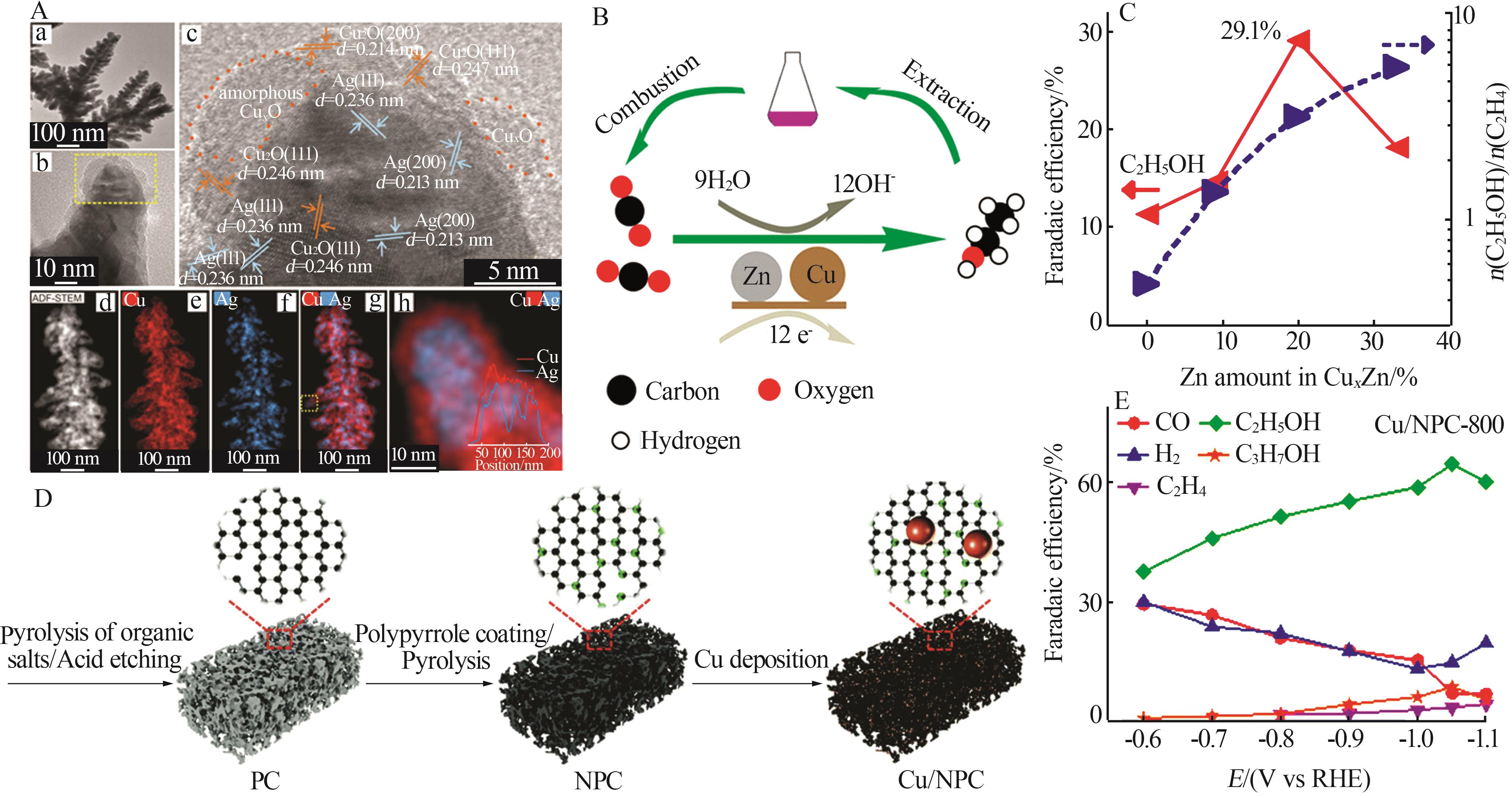
| Ag-Co[ | -0.80 | 72.3 | 7.40 |
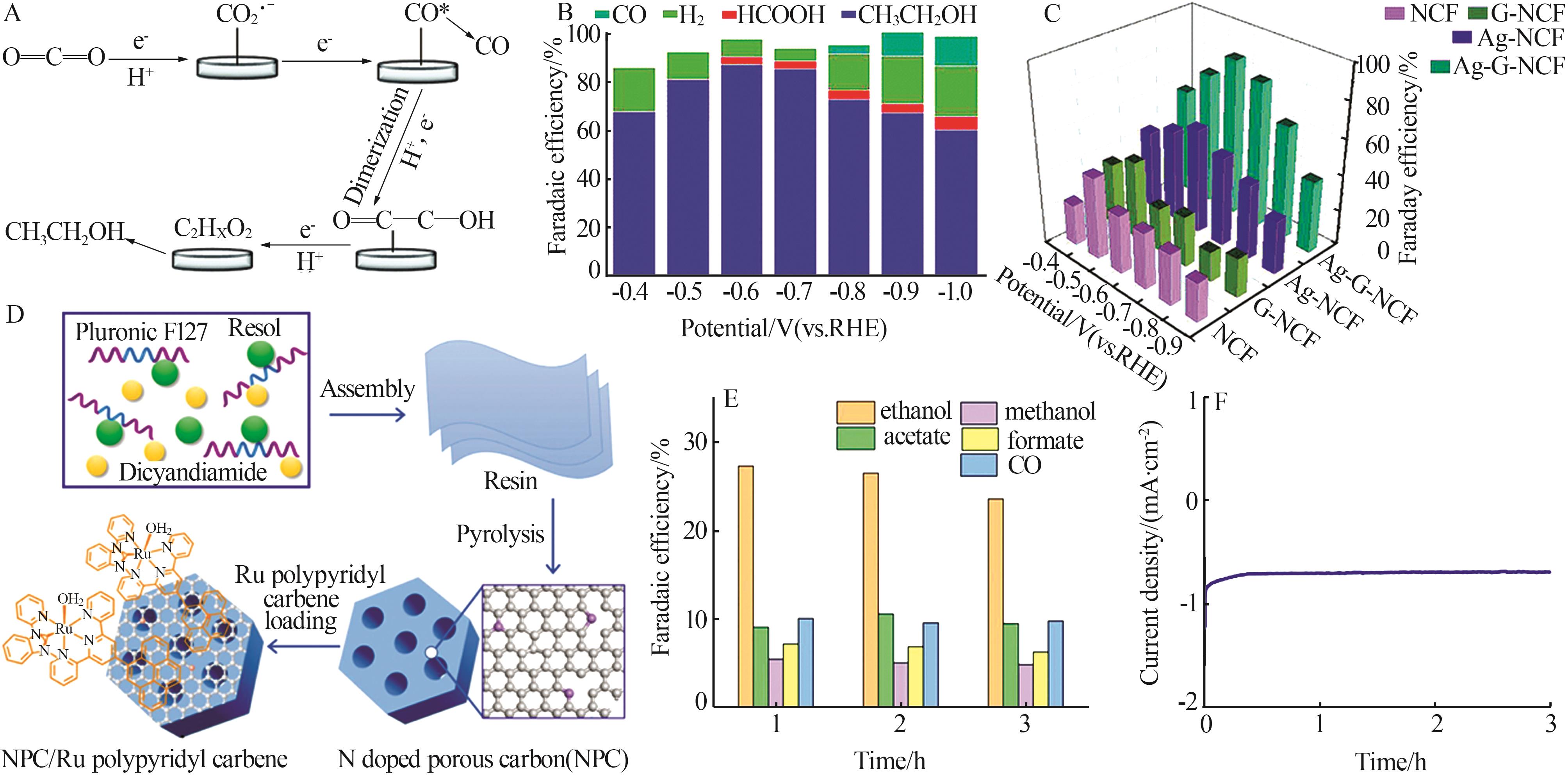
| Cu2S@Cu(V) [ | -0.95 | 23.1 | — |
| Cu(N-C/Cu) [ | -0.68 | 52±1 | 156±13 |
| Au@Cu2O[ | -0.30 | 52.3 | — |
| SnS2/Sn1-O3G[ | -0.90 | 97.0 | 17.80 |
| 1 | DINH C T, BURDYNY T, KIBRIA M G,et al.CO2 electroreduction to ethylene via hydroxide⁃mediated copper catalysis at an ab⁃ rupt interface[J].Science,2018,360(6390):783-787. |
| 2 | NITOPI S, BERTHEUSSEN E, SCOTT S B,et al.Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte[J].Chemical Reviews,2019,119(12):7610-7672. |
| 3 | BIRDJA Y Y, PÉREZ-GALLENT E, FIGUEIREDO M C,et al.Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels[J].Nature Energy,2019,4:732-745. |
| 4 | DE LUNA P, HAHN C, HIGGINS D,et al.What would it take for renewably powered electrosynthesis to displace petrochemical processes?[J].Science,2019,364(6438):eaav3506. |
| 5 | XU Haiping, REBOLLAR D, HE Haiying,et al.Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper[J].Nature Energy,2020,5:623-632. |
| 6 | ZHANG Xiaoyu, XUE Dongping, JIANG Su,et al.Rational confinement engineering of MOF-derived carbonbased electrocatalysts toward CO2 reduction and O2 reduction reactions[J].InfoMat,2022(3):34-63. |
| 7 | 苑琦,杨昊,谢淼,等.二氧化碳电还原反应的理论研究[J].物理化学学报,2021,37(5):169-182. |
| YUAN Qi, YANG Hao, XIE Miao,et al.Theoretical research on the electroreduction of carbon dioxide[J].Acta Physico-Chimica Sinica,2021,37(5):169-182. | |
| 8 | HORI Y, TAKAHASHI I, KOGA O,et al.Electrochemical reduction of carbon dioxide at various series of copper single crystal electrodes[J].Journal of Molecular Catalysis A:Chemical,2003,199(1/2):39-47. |
| 9 | GAO Dunfeng, ARÁN-AIS R M, JEON H S,et al.Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products[J].Nature Catalysis,2019,2:198-210. |
| 10 | JI Lei, CHANG Le, ZHANG Ya,et al.Electrocatalytic CO2 reduction to alcohols with high selectivity over a two⁃dimensional Fe2P2S6 nanosheet[J].ACS Catalysis,2019,9(11):9721-9725. |
| 11 | LUO Wenjia, NIE Xiaowa, JANIK M J,et al.Facet dependence of CO2 reduction paths on Cu electrodes[J].ACS Catalysis,2016,6(1):219-229. |
| 12 | HORI Y.Electrochemical CO2 reduction on metal electrodes[M].New York:Springer,2008:89-189. |
| 13 | SONG Yang, PENG Rui, HENSLEY D K,et al.High⁃selectivity electrochemical conversion of CO2 to ethanol using a copper nanoparticle/N-doped graphene electrode[J].ChemistrySelect,2016,1(19):6055-6061. |
| 14 | YUAN Jing, YANG Manping, HU Qiaoli,et al.Cu/TiO2 nanoparticles modified nitrogen⁃doped graphene as a highly efficient catalyst for the selective electroreduction of CO2 to different alcohols[J].Journal of CO2 Utilization,2018,24:334-340. |
| 15 | DU Juan, LI Shaopeng, LIU Shulin,et al.Selective electrochemical reduction of carbon dioxide to ethanol via a relay catalytic platform[J].Chemical Science,2020,11(19):5098-5104. |
| 16 | MONTOYA J H, SHI Chuan, CHAN Karen,et al.Theoretical insights into a CO dimerization mechanism in CO2 electroreducti⁃on[J].The Journal of Physical Chemistry Letters,2015,6(11):2032-2037. |
| 17 | LIU Xinyan, XIAO Jianping, PENG Hongjie,et al.Understanding trends in electrochemical carbon dioxide reduction rates[J].Nature Communications,2017,8:15438. |
| 18 | JOUNY M, LUC W, JIAO Feng.High⁃rate electroreduction of carbon monoxide to multi⁃carbon products[J].Nature Catalysis,2018,1:748-755. |
| 19 | GU Zhengxiang, SHEN Hao, CHEN Zheng,et al.Efficient electrocatalytic CO2 reduction to C2+ alcohols at defect⁃site⁃rich Cu surface[J].Joule,2021,5(2):429-440. |
| 20 | LI C W, CISTON J, KANAN M W.Electroreduction of carbon monoxide to liquid fuel on oxide⁃derived nanocrystalline copp⁃ er[J].Nature,2014,508:504-507. |
| 21 | CUELLAR N R, WIESNER-FLEISCHER K, FLEISCHER M,et al.Advantages of CO over CO2 as reactant for electrochemical reduction to ethylene,ethanol and n-propanol on gas diffusion electrodes at high current densities[J].Electrochimica Acta,2019,307:164-175. |
| 22 | WANG Xingli, DE ARAÚJO J F, JU Wen,et al.Mechanistic reaction pathways of enhanced ethylene yields during electroreduction of CO2-CO co⁃feeds on Cu and Cu-tandem electrocataly⁃sts[J].Nature Nanotechnology,2019,14:1063-1070. |
| 23 | TING L R L, PIQUÉ O, LIM S Y,et al.Enhancing CO2 electroreduction to ethanol on copper⁃silver composites by opening an alternative catalytic pathway[J].ACS Catalysis,2020,10(7):4059-4069. |
| 24 | IYENGAR P, KOLB M J, PANKHURST J R,et al.Elucidating the facet⁃dependent selectivity for CO2 electroreduction to ethanol of Cu-Ag tandem catalysts[J].ACS Catalysis,2021,11(8):4456-4463. |
| 25 | WEN Guobin, REN Bohua, ZHENG Yun,et al.Engineering electrochemical surface for efficient carbon dioxide upgrade[J].Advanced Energy Materials,2022,12(3):2103289. |
| 26 | DUTTA A, MONTIEL I Z, ERNI R,et al.Activation of bimetallic AgCu foam electrocatalysts for ethanol formation from CO2 by selective Cu oxidation/reduction[J].Nano Energy,2020,68:104331. |
| 27 | MORALES-GUIO C G, CAVE E R, NITOPI S A,et al.Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst[J].Nature Catalysis,2018,1:764-771. |
| 28 | SHEN Sibo, PENG Xianyun, SONG Lida,et al.AuCu alloy nanoparticle embedded Cu submicrocone arrays for selective conversion of CO2 to ethanol[J].Small,2019,15(37):e1902229. |
| 29 | REN Dan, ANG B S H, YEO B S.Tuning the selectivity of carbon dioxide electroreduction toward ethanol on oxide⁃derived Cu x Zn catalysts[J].ACS Catalysis,2016,6(12):8239-8247. |
| 30 | LV Kuilin, FAN Yanchen, ZHU Ying,et al.Elastic Ag-anchored N-doped graphene/carbon foam for the selective electrochemical reduction of carbon dioxide to ethanol[J].Journal of Materials Chemistry A,2018,6(12):5025-5031. |
| 31 | LIU Yanming, ZHANG Yujing, CHENG Kai,et al.Selective electrochemical reduction of carbon dioxide to ethanol on a boron⁃and nitrogen⁃co⁃doped nanodiamond[J].Angewandte Chemie International Edition,2017,56(49):15607-15611. |
| 32 | LIU Yanming, FAN Xinfei, NAYAK A,et al.Steering CO2 electroreduction toward ethanol production by a surface⁃bound Ru polypyridyl carbene catalyst on N-doped porous carbon[J].Proceedings of the National Academy of Sciences of the United States of America,2019,116(52):26353-26358. |
| 33 | ZHANG Qiang, TAO Shuihui, DU Jun,et al.A cold plasma⁃activated in situ AgCo surface alloy for enhancing the electroreduction of CO2 to ethanol[J].Journal of Materials Chemistry A,2020,8(17):8410-8420. |
| 34 | ZHUANG Taotao, LIANG Zhiqin, SEIFITOKALDANI A,et al.Steering post-C-C coupling selectivity enables high efficiency electroreduction of carbon dioxide to multi⁃carbon alcohols[J].Nature Catalysis,2018,1:421-428. |
| 35 | WANG Xue, WANG Ziyun, GARCÍA DE ARQUER F P,et al.Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation[J].Nature Energy,2020,5:478-486. |
| 36 | ZHANG Binbin, WANG Yahui, XU Shanmin,et al.Tuning nanocavities of Au@Cu2O yolk⁃shell nanoparticles for highly selective electroreduction of CO2 to ethanol at low potential[J].RSC Advances,2020,10(33):19192-19198. |
| 37 | DING Jie, YANG Hongbin, MA Xuelu,et al.A tin⁃based tandem electrocatalyst for CO2 reduction to ethanol with 80% selectivi⁃ ty[J].Nature Energy,2023,8:1386-1394. |
| 38 | THEAKER N, STRAIN J M, KUMAR B,et al.Heterogeneously catalyzed two⁃step cascade electrochemical reduction of CO2 to ethanol[J].Electrochimica Acta,2018,274:1-8. |
| 39 | ROMERO CUELLAR N S, SCHERER C, KAÇKAR B,et al.Two⁃step electrochemical reduction of CO2 towards multi⁃carbon products at high current densities[J].Journal of CO2 Utilization,2020,36:263-275. |
| 40 | WU Guoling, SONG Yaru, ZHENG Qiang,et al.Selective electroreduction of CO2 to n-propanol in two⁃step tandem catalytic system[J].Advanced Energy Materials,2022,12(36):2202054. |
| 41 | ZHANG Tianyu, BUI J C, LI Zhengyuan,et al.Highly selective and productive reduction of carbon dioxide to multicarbon products via in situ CO management using segmented tandem electrodes[J].Nature Catalysis,2022,5:202-211. |
| 42 | HAN Zhiji, KORTLEVER R, CHEN H Y,et al.CO2 reduction selective for C≥2 products on polycrystalline copper with N-substi⁃tuted pyridinium additives[J].ACS Central Science,2017,3(8):853-859. |
| 43 | HUANG Yun, HANDOKO A D, HIRUNSIT P,et al.Electrochemical reduction of CO2 using copper single⁃crystal surfaces:Effects of CO* coverage on the selective formation of ethylene[J].ACS Catalysis,2017,7(3):1749-1756. |
| 44 | Fang LÜ, BAO Haihong, MI Yuying,et al.Electrochemical CO2 reduction:From nanoclusters to single atom catalysts[J].Sustainable Energy & Fuels,2020,4(3):1012-1028. |
| 45 | GOODPASTER J D, BELL A T, HEAD-GORDON M.Identification of possible pathways for C-C bond formation during electrochemical reduction of CO2:New theoretical insights from an improved electrochemical model[J].The Journal of Physical Chemistry Letters,2016,7(8):1471-1477. |
| 46 | KIM Y, PARK S, SHIN S J,et al.Time⁃resolved observation of C-C coupling intermediates on Cu electrodes for selective electrochemical CO2 reduction[J].Energy & Environmental Science,2020,13(11):4301-4311. |
| 47 | XIAO Hai, CHENG Tao, W A Ⅲ GODDARD,et al.Mechanistic explanation of the pH dependence and onset potentials for hydrocarbon products from electrochemical reduction of CO on Cu (111)[J].Journal of the American Chemical Society,2016,138(2):483-486. |
| 48 | XIAO Hai, CHENG Tao, W A Ⅲ GODDARD.Atomistic mechanisms underlying selectivities in C(1) and C(2) products from electrochemical reduction of CO on Cu(111)[J].Journal of the American Chemical Society,2017,139(1):130-136. |
| 49 | LIU Xinyan, SCHLEXER P, XIAO Jianping,et al.pH effects on the electrochemical reduction of CO2 towards C2 products on stepped copper[J].Nature Communications,2019,10:32. |
| 50 | GARZA A J, BELL A T, HEAD-GORDON M.Mechanism of CO2 reduction at copper surfaces:Pathways to C2 products[J].ACS Catalysis,2018,8(2):1490-1499. |
| 51 | LEDEZMA-YANEZ I, GALLENT E P, KOPER M T M,et al.Structure⁃sensitive electroreduction of acetaldehyde to ethanol on copper and its mechanistic implications for CO and CO2 reduction[J].Catalysis Today,2016,262:90-94. |
| 52 | CHENG Tao, XIAO Hai, W A Ⅲ GODDARD.Full atomistic reaction mechanism with kinetics for CO reduction on Cu(100) from ab initio molecular dynamics free⁃energy calculations at 298 K[J].Proceedings of the National Academy of Sciences of the United States of America,2017,114(8):1795-1800. |
| 53 | HANSELMAN S, KOPER M T M, CALLE-VALLEJO F.Computational comparison of late transition metal(100) surfaces for the electrocatalytic reduction of CO to C2 species[J].ACS Energy Letters,2018,3(5):1062-1067. |
| 54 | FAN Qun, ZHANG Mingli, JIA Mingwen,et al.Electrochemical CO2 reduction to C2+ species:Heterogeneous electrocatalysts,reaction pathways,and optimization strategies[J].Materials Today Energy,2018,10:280-301. |
| 55 | XIANG Kaisong, SHEN Fenghua, FU Yingxue,et al.Boosting CO2 electroreduction towards C2+ products via CO* intermediate manipulation on copper⁃based catalysts[J].Environmental Science:Nano,2022,9(3):911-953. |
| 56 | FU Xianbiao, ZHANG Jiahao, KANG Yijin.Electrochemical reduction of CO2 towards multi⁃carbon products via a two⁃step process[J].Reaction Chemistry & Engineering,2021,6(4):612- 628. |
| 57 | WU Jingjie, LIU Mingjie, SHARMA P P,et al.Incorporation of nitrogen defects for efficient reduction of CO2 via two⁃electron pathway on three⁃dimensional graphene foam[J].Nano Letters,2016,16(1):466-470. |
| 58 | LIU Song, YANG Hongbin, HUANG Xiang,et al.Identifying active sites of nitrogen⁃doped carbon materials for the CO2 reduction reaction[J].Advanced Functional Materials,2018,28(21):1800499. |
| 59 | HURSÁN D, SAMU A A, JANOVÁK L,et al.Morphological attributes govern carbon dioxide reduction on N-doped carbon electrodes[J].Joule,2019,3(7):1719-1733. |
| 60 | HAN H,NOH Y, KIM Y,et al.Selective electrochemical CO2 conversion to multicarbon alcohols on highly efficient N-doped porous carbon⁃supported Cu catalysts[J].Green Chemistry,2020, 22(1):71-84. |
| 61 | MEDFORD A J, VOJVODIC A, HUMMELSHØJ J S,et al.From the Sabatier principle to a predictive theory of transition⁃metal heterogeneous catalysis[J].Journal of Catalysis,2015,328:36- 42. |
| 62 | ZHANG Xiaguang, JIN Xi, WU Deyin,et al.Selective electrocatalytic mechanism of CO2 reduction reaction to CO on silver electrodes:A unique reaction intermediate[J].The Journal of Physical Chemistry C,2018,122(44):25447-25455. |
| 63 | POPOVIĆ S, SMILJANIĆ M, JOVANOVIČ P,et al.Stability and degradation mechanisms of copper⁃based catalysts for electrochemical CO2 reduction[J].Angewandte Chemie,2020,59(35):14736-14746. |
| 64 | CHANG Zhiyuan, HUO Shengjuan, HE Jinmei,et al.Facile synthesis of Cu-Ag bimetallic electrocatalyst with prior C2 products at lower overpotential for CO2 electrochemical reduction[J].Surfaces and Interfaces,2017,6:116-121. |
| 65 | WU Jingjie, MA Sichao, SUN Jing,et al.A metal⁃free electrocatalyst for carbon dioxide reduction to multi⁃carbon hydrocarbons and oxygenates[J].Nature Communications,2016,7:13869. |
| 66 | VARANDILI S, HUANG Jianfeng, OVEISI E,et al.Synthesis of Cu/CeO2- x nanocrystalline heterodimers with interfacial active sites to promote CO2 electroreduction[J].ACS Catalysis,2019,9(6):5035-5046. |
| 67 | DUTTA A, RAHAMAN M, LUEDI N C,et al.Morphology matters:Tuning the product distribution of CO2 electroreduction on oxide⁃derived Cu foam catalysts[J].ACS Catalysis,2016,6(6):3804-3814. |
| 68 | O’MARA P B, WILDE P, BENEDETTI T M,et al.Cascade reactions in nanozymes:Spatially separated active sites inside Ag-core⁃porous-Cu-shell nanoparticles for multistep carbon dioxide reduction to higher organic molecules[J].Journal of the American Chemical Society,2019,141(36):14093-14097. |
| 69 | CHEN L D, URUSHIHARA M, CHAN K,et al.Electric field effects in electrochemical CO2 reduction[J].ACS Catalysis,2016, 6(10):7133-7139. |
| 70 | JANSONIUS R P, REID L M, VIRCA C N,et al.Strain engineering electrocatalysts for selective CO2 reduction[J].ACS Energy Letters,2019,4(4):980-986. |
| 71 | LI Xiaotong, WU Xiuju, LV Xiangzhou,et al.Recent advances in metal⁃based electrocatalysts with hetero⁃interfaces for CO2 reduction reaction[J].Chem Catalysis,2022,2(2):262-291. |
| 72 | GILROY K D, RUDITSKIY A, PENG H C,et al.Bimetallic nanocrystals:Syntheses,properties,and applications[J].Chemical Reviews,2016,116(18):10414-10472. |
| 73 | DUTTA A, RAHAMAN M, MOHOS M,et al.Electrochemical CO2 conversion using skeleton(sponge) type of Cu catalysts[J].ACS Catalysis,2017,7(8):5431-5437. |
| 74 | CAI Rongming, SUN Mingzi, YANG Fei,et al.Engineering Cu(I)/Cu(0) interfaces for efficient ethanol production from CO2 electroreduction[J].Chem,2024,10(1):211-233. |
| 75 | LEES E W, MOWBRAY B A W, PARLANE F G L,et al.Gas diffusion electrodes and membranes for CO2 reduction electrolyse⁃rs[J].Nature Reviews Materials,2022,7:55-64. |
| 76 | 毛庆,赵健,刘松,等.Ni单原子催化剂表面CO2电还原动力学的电化学谱学解析[J].高等学校化学学报,2020,41(5):1058-1067. |
| MAO Qing, ZHAO Jian, LIU Song,et al.Electrochemical spectroscopy analysis for kinetics of the CO2 electroreduction reaction on Ni single atom catalysts[J].Chemical Journal of Chinese Universities,2020,41(5):1058-1067. | |
| 77 | FENG Haisong, DING Hu, HE Peinan,et al.Data⁃driven design of dual⁃metal⁃site catalysts for the electrochemical carbon dioxide reduction reaction[J].Journal of Materials Chemistry A,2022,10(36):18803-18811. |
| [1] | YAO Jiankang, HU Shuozhen, NIU Dongfang, WU Jianping, ZHANG Xinsheng. Study on electrochemical treatment of sodium chloride organic waste salt in spice industry [J]. Inorganic Chemicals Industry, 2024, 56(3): 105-115. |
| [2] | MA Lianren, XIE Hongyan. Study on preparation of LiMn0.7Fe0.3PO4/C cathode materials by two-step solid-phase method with surfactant [J]. Inorganic Chemicals Industry, 2024, 56(11): 39-44. |
| [3] | KANG Le, JING Maoxiang, LI Donghong, HU Xinyu, JIA Chunyan. Study on preparation and electrochemical performance of lithium aluminate nanorods modified solid electrolyte [J]. Inorganic Chemicals Industry, 2023, 55(8): 65-70. |
| [4] | Wang Shidong,Ye Xiushen,Li Quan,Huo Yan,Li Mingzhen,Zhang Huifang,Pang Quanshi,Wu Zhijian. Electrochemcial behavior of impurity elements in lithium electrolysis [J]. Inorganic Chemicals Industry, 2021, 53(4): 52-55. |
| [5] | Wu Jing,Ren Xiulian,Wei Qifeng. Research progress on separation and extraction of lithium from salt-lake brine [J]. Inorganic Chemicals Industry, 2020, 52(12): 1-6. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||