Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (11): 55-59.doi: 10.19964/j.issn.1006-4990.2021-0342
• Research & Development • Previous Articles Next Articles
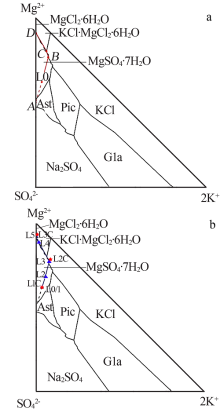
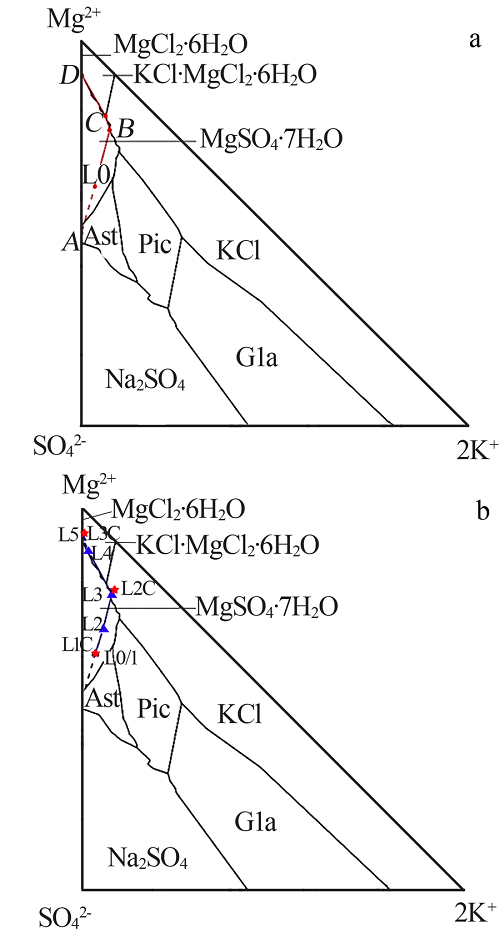
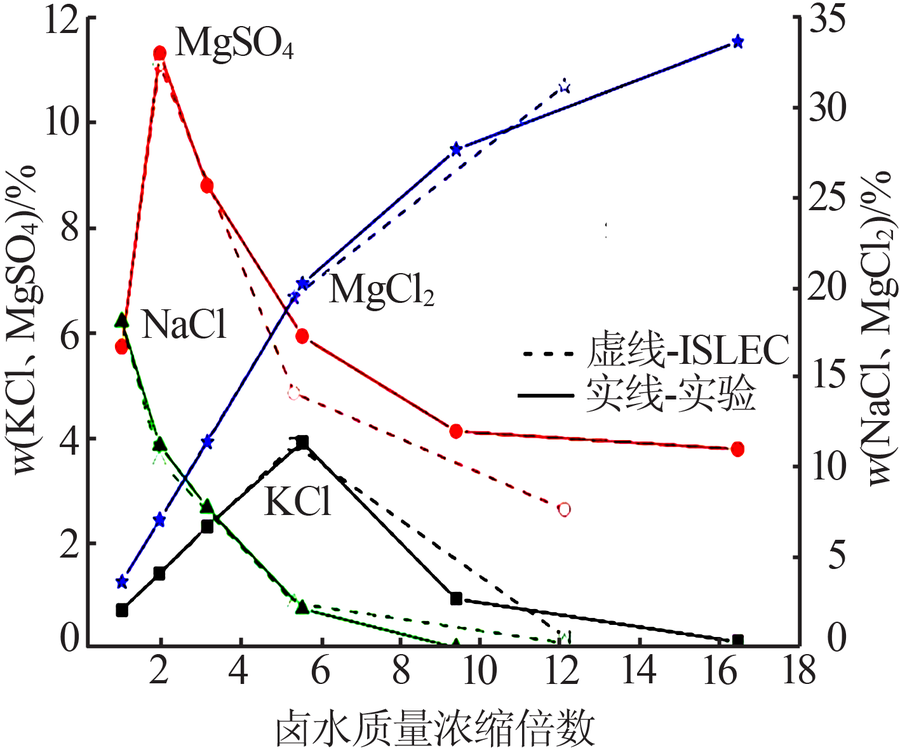
Crystallizing sequence of salts and enrichment rule of boron and lithium during natural evaporation of Manasi salt lake brine
BIAN Shaoju1( ), XU Naicai1, ZHANG Yongxing2, LI Wu3(
), XU Naicai1, ZHANG Yongxing2, LI Wu3( )
)
- 1. School of Chemistry and Chemical Engineering,Qinghai Normal University,Xining 810016,China
2. Zhengzhou Institute of Multipurpose Utilization of Mineral Resources,CAGS
3. Qinghai Institute of Salt Lakes,CAS