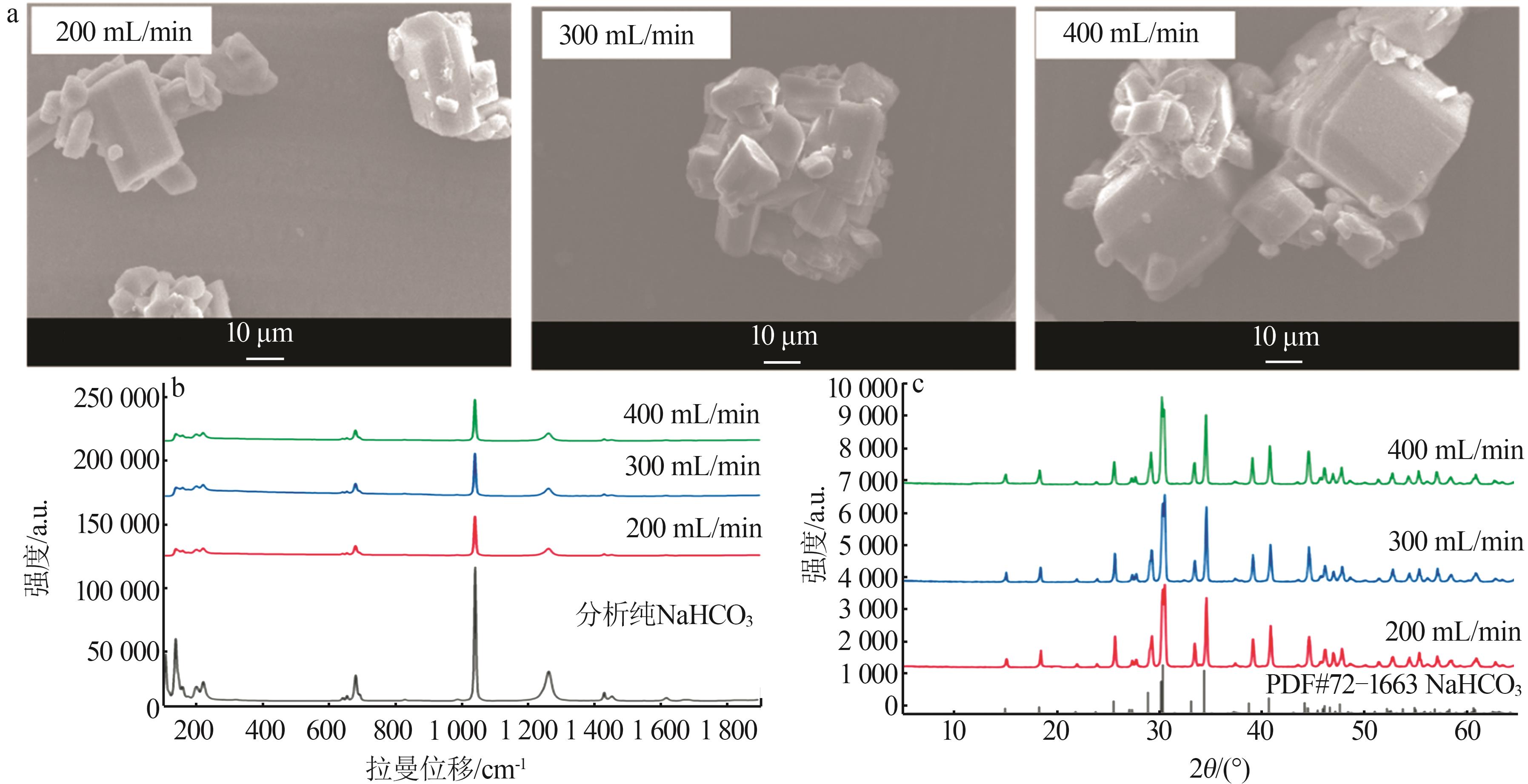
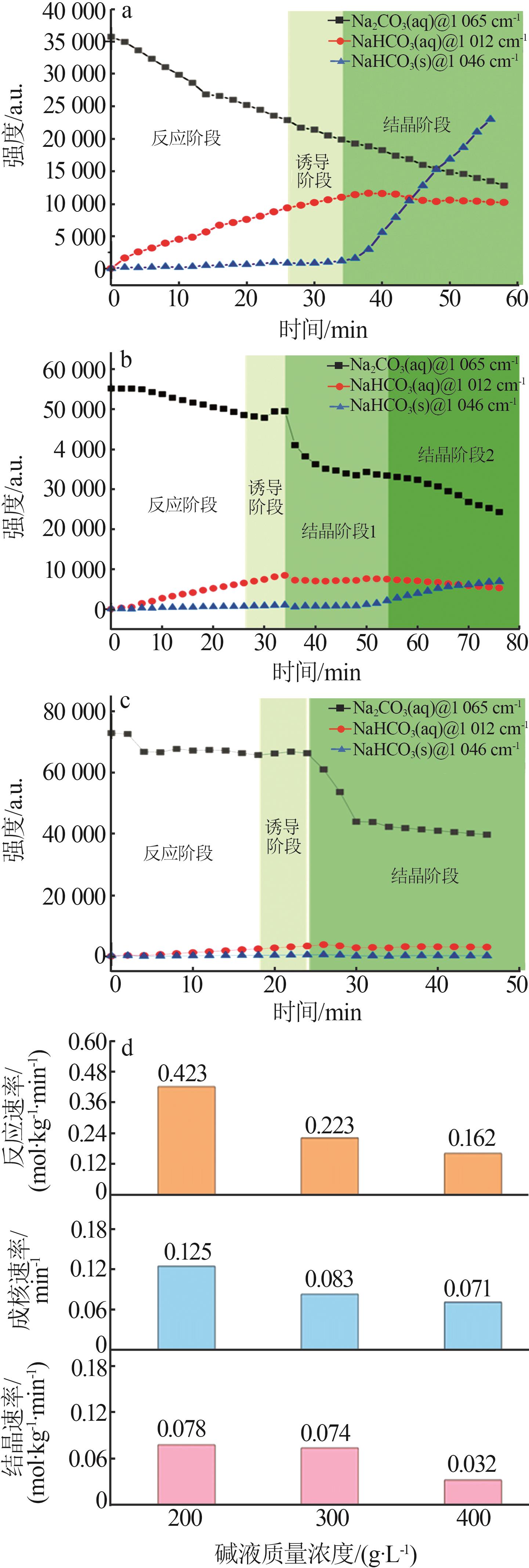
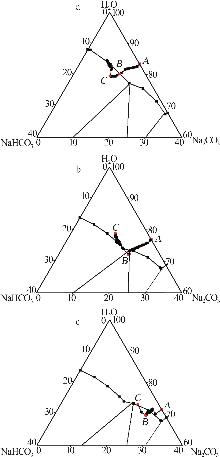
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (10): 55-63.doi: 10.19964/j.issn.1006-4990.2024-0550
• Research & Development • Previous Articles Next Articles
Study on reaction crystallization process of soda ash and carbon dioxide
ZHANG Menghan1( ), DU Wei1, XU Quan2, CAO Jun2, TANG Na1(
), DU Wei1, XU Quan2, CAO Jun2, TANG Na1( )
)
- 1. College of Chemical Engineering and Materials Science,Tianjin University of Science & Technology,Tianjin 300457,China
2. Jiangsu Suyanjingshen Co. ,Ltd. ,Huai′an 223200,China
-
Received:2024-10-15Online:2025-10-10Published:2025-05-13 -
Contact:TANG Na E-mail:2289151500@qq.com;tjtangna@tust.edu.cn
CLC Number:
Cite this article
ZHANG Menghan, DU Wei, XU Quan, CAO Jun, TANG Na. Study on reaction crystallization process of soda ash and carbon dioxide[J]. Inorganic Chemicals Industry, 2025, 57(10): 55-63.
share this article
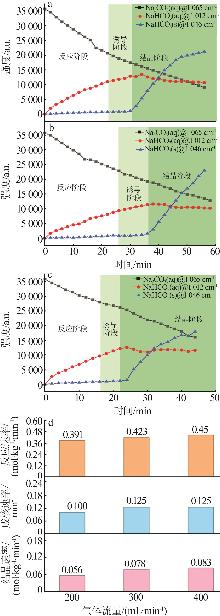
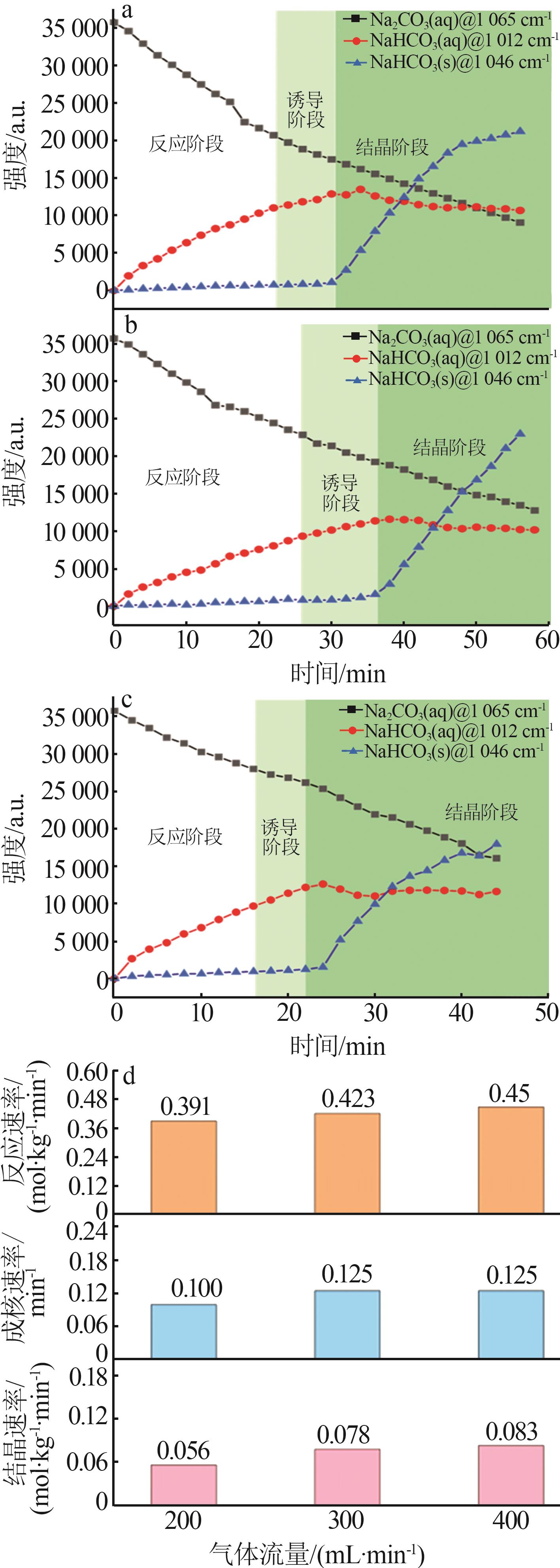
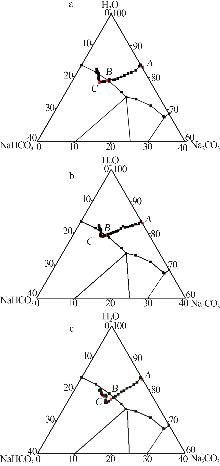
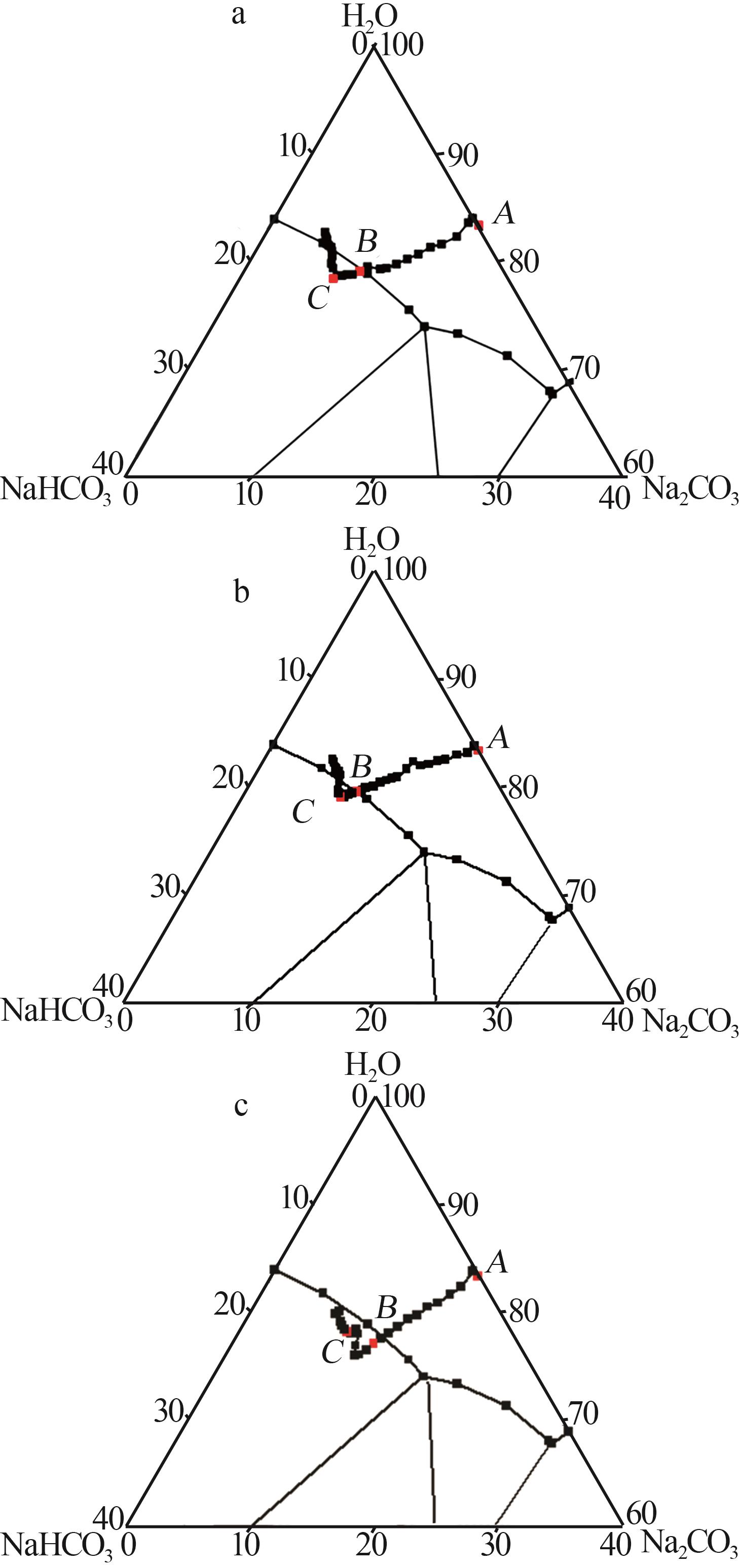
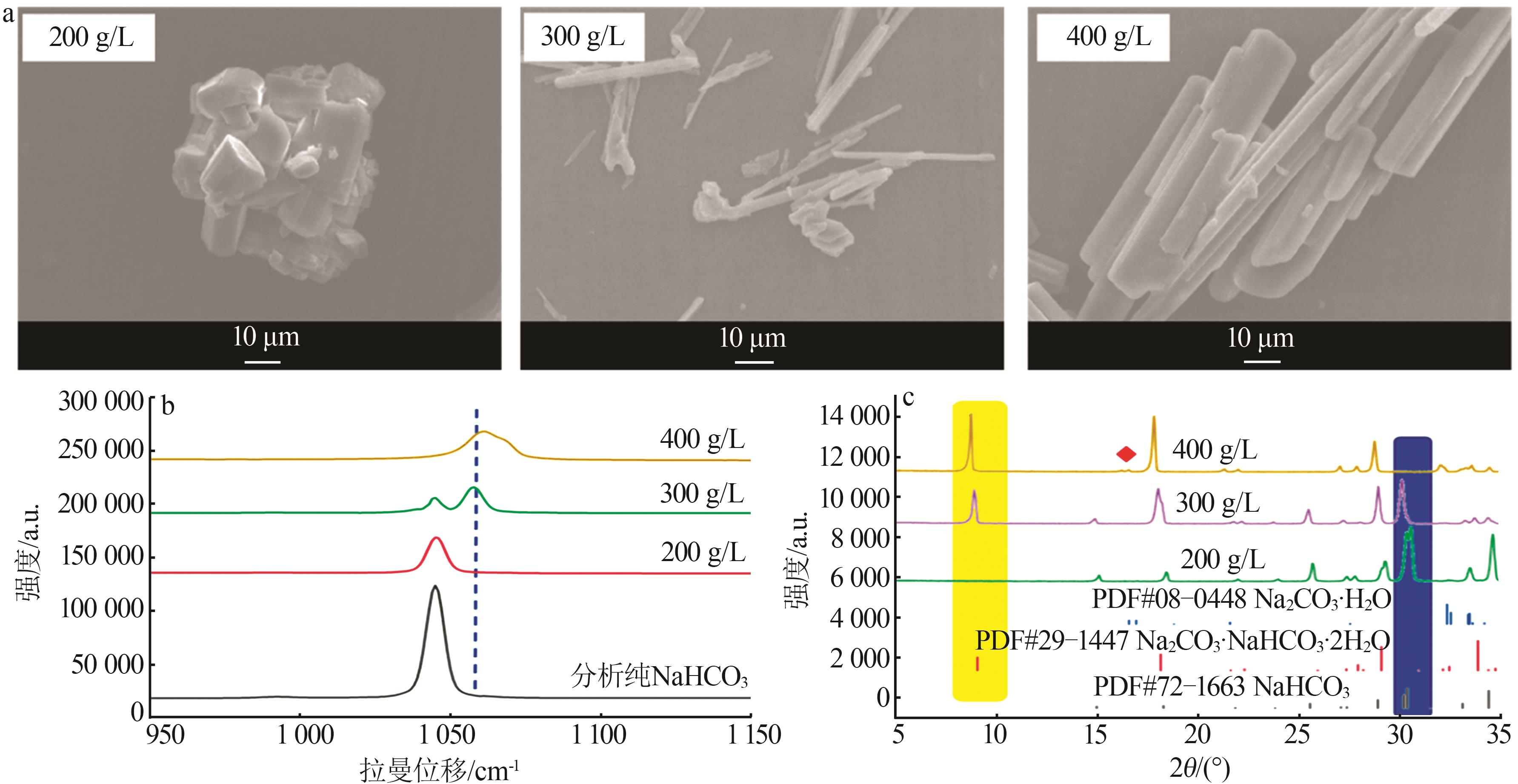
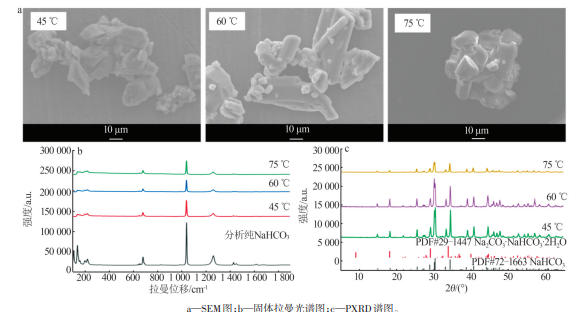
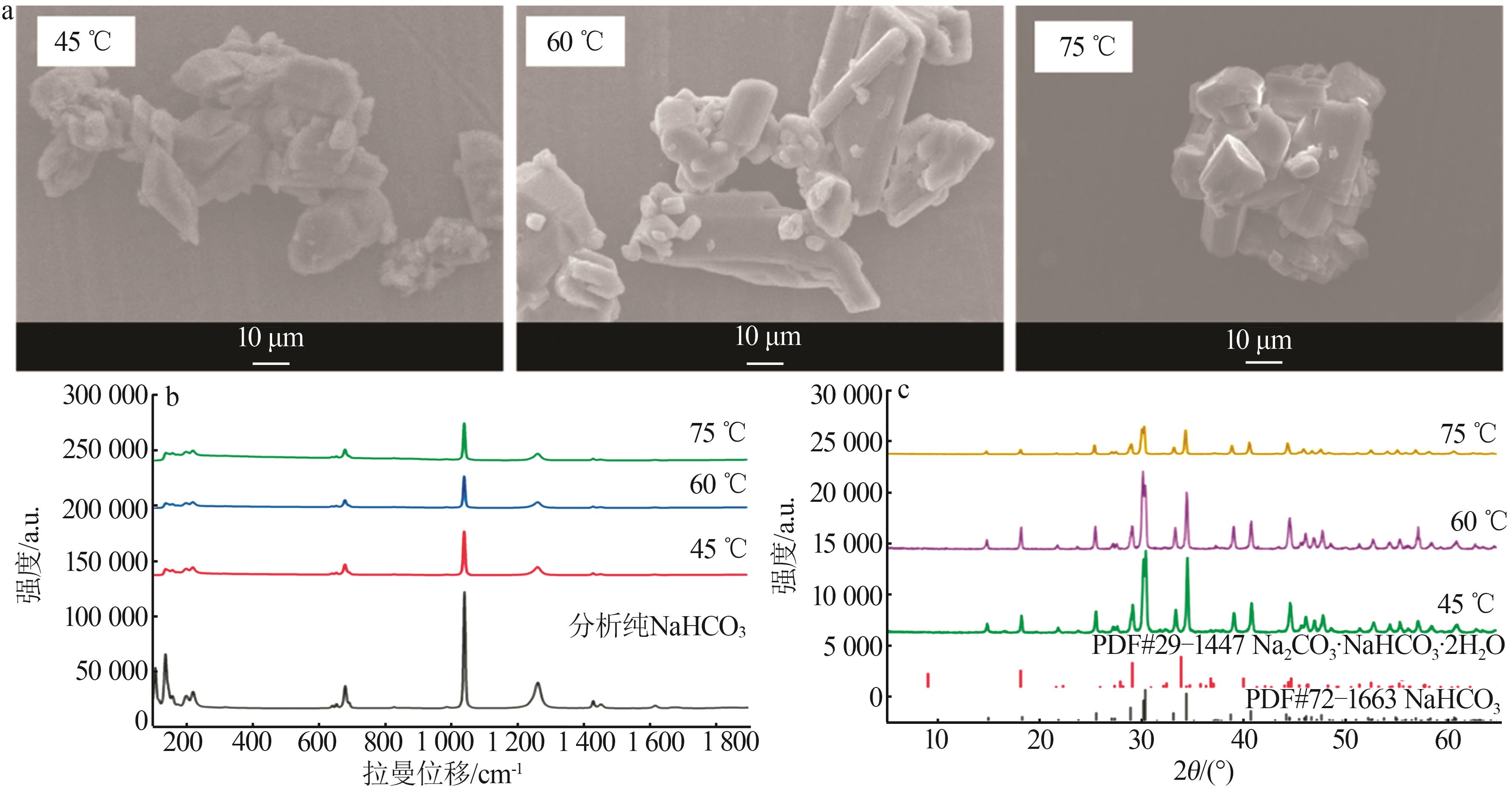
Table 1
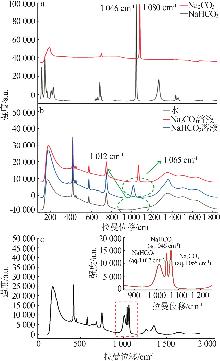
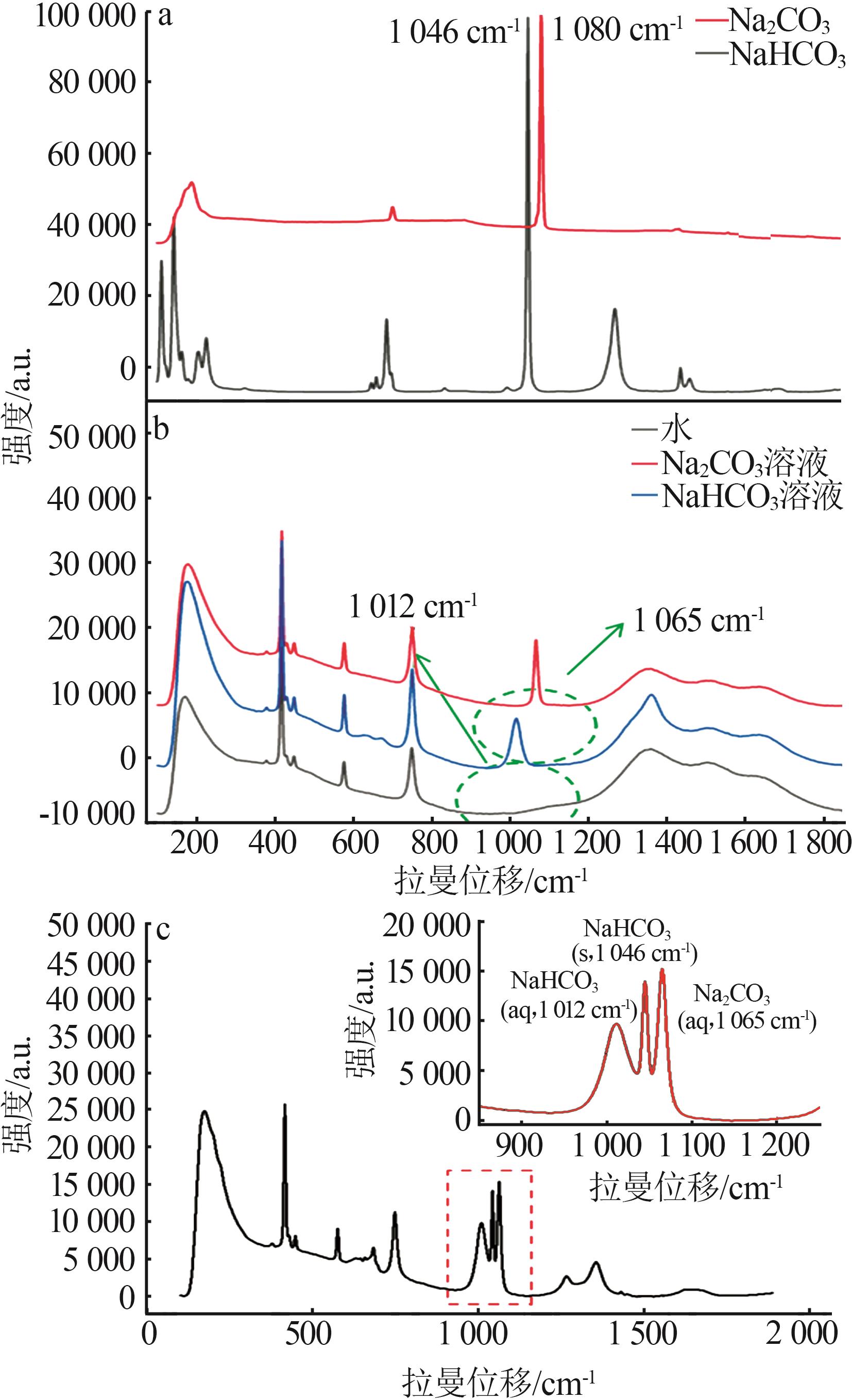
Standard curves of concentration and Raman spectra intensity for Na2CO3 and NaHCO3 at different temperatures"
| 温度/℃ | 曲线方程 | R2 | |
|---|---|---|---|
| 45 | Na2CO3 | y=198.4x-1 270.1 | 0.994 1 |
| NaHCO3 | y=79.749x-54.878 | 0.998 7 | |
| 60 | Na2CO3 | y=111.95x+989.24 | 0.997 4 |
| NaHCO3 | y=46.555x-132.28 | 0.998 3 | |
| 75 | Na2CO3 | y=74.001x-126.3 | 0.999 4 |
| NaHCO3 | y=89.414x-219.02 | 0.999 6 | |
| [1] | 王将,胡东岭,江德发,等.一种监控纯碱煅烧工序电除尘器效率的方法[J].纯碱工业,2019(4):7-10. |
| WANG Jiang, HU Dongling, JIANG Defa,et al.Method for inspecting the efficiency of the electric precipitator in soda calcination process[J].Soda Industry,2019(4):7-10. | |
| [2] | 中昊(大连)化工研究设计院有限公司.纯碱工学[M].3版.北京:化学工业出版社,2014. |
| [3] | 乔明,周立峰,李清春.国内外天然碱加工工艺研究进展[J].盐科学与化工,2024,53(10):1-4. |
| QIAO Ming, ZHOU Lifeng, LI Qingchun.Research progress in natural alkali processing technology at home and abroad[J].Journal of Salt Science and Chemical Industry,2024,53(10):1-4. | |
| [4] | RAO A R, TIDE P S, GEORGE B K,et al.Quasi-dynamic model for dissolution coupled with reaction and precipitation of sodium bicarbonate in fed-batch reactive crystallization[J].Chemical Engineering Journal Advances,2023,15:100504. |
| [5] | 马海忠.小苏打生产工艺中影响总碱量的因素[J].盐科学与化工,2020,49(6):28-29,33. |
| MA Haizhong.Factors affecting the total alkali content in the production process of baking soda[J].Journal of Salt Science and Che- | |
| Industry mical,2020,49(6):28-29,33. | |
| [6] | 赵贵乔,谢智勇,王松晓.小苏打生产工艺优化提升研究[Z].天津:天津渤化永利化工股份有限公司,2022. |
| [7] | JIANG Shifeng, ZHANG Yuande, LI Zhibao.A new industrial process of NaHCO3 and its crystallization kinetics by using the common ion effect of Na2CO3 [J].Chemical Engineering Journal,2019,360:740-749. |
| [8] | 轩辕书天.纯碱碳化法制备大颗粒碳酸氢钠过程研究[D].天津:天津大学,2022. |
| XUANYUAN Shutian.Study on the process of preparing large particle sodium bicarbonate by sodium carbonate carbonization[D].Tianjin:Tianjin University,2022. | |
| [9] | CLERCQ S, CRAMPON C, BADENS E.Atypical crystal growth within the supercritical antisolvent process:Experimental and molecular modeling approach with sodium bicarbonate[J].The Journal of Supercritical Fluids,2024,207:106188. |
| [10] | 范海荣,林鹏鹏,宫明明,等.低盐小苏打生产的水平衡控制[J].纯碱工业,2024(4):44-46. |
| FAN Hairong, LIN Pengpeng, GONG Mingming,et al.Water balance control in the production of low salt baking soda[J].Soda Industry,2024(4):44-46. | |
| [11] | 徐建华,罗飞跃,赵沁乐,等.浅谈二氧化碳浓度对碳化结晶过程的影响与工艺改进[J].纯碱工业,2024(3):3-6. |
| XU Jianhua, LUO Feiyue, ZHAO Qinyue,et al.Influence of carbon dioxide concentration on the carbonization crystallization process and process improvement[J].Soda Industry,2024(3):3-6. | |
| [12] | 沈爱国,梁伟伟,麦家铭.一种测定固体样品中的碳酸钠或碳酸氢钠含量的方法:CN,106950215A[P].2017-07-14. |
| [13] | 邹晓艳,吕新彪,何谋春.常见酸根离子浓度的激光拉曼光谱定量分析[J].岩矿测试,2007,26(1):26-28. |
| ZOU Xiaoyan, Xinbiao LÜ, HE Mouchun.Quantitative analysis of common acid radical ions by laser Raman spectrometry[J].Rock and Mineral Analysis,2007,26(1):26-28. | |
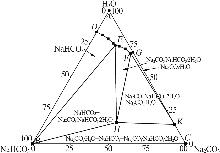
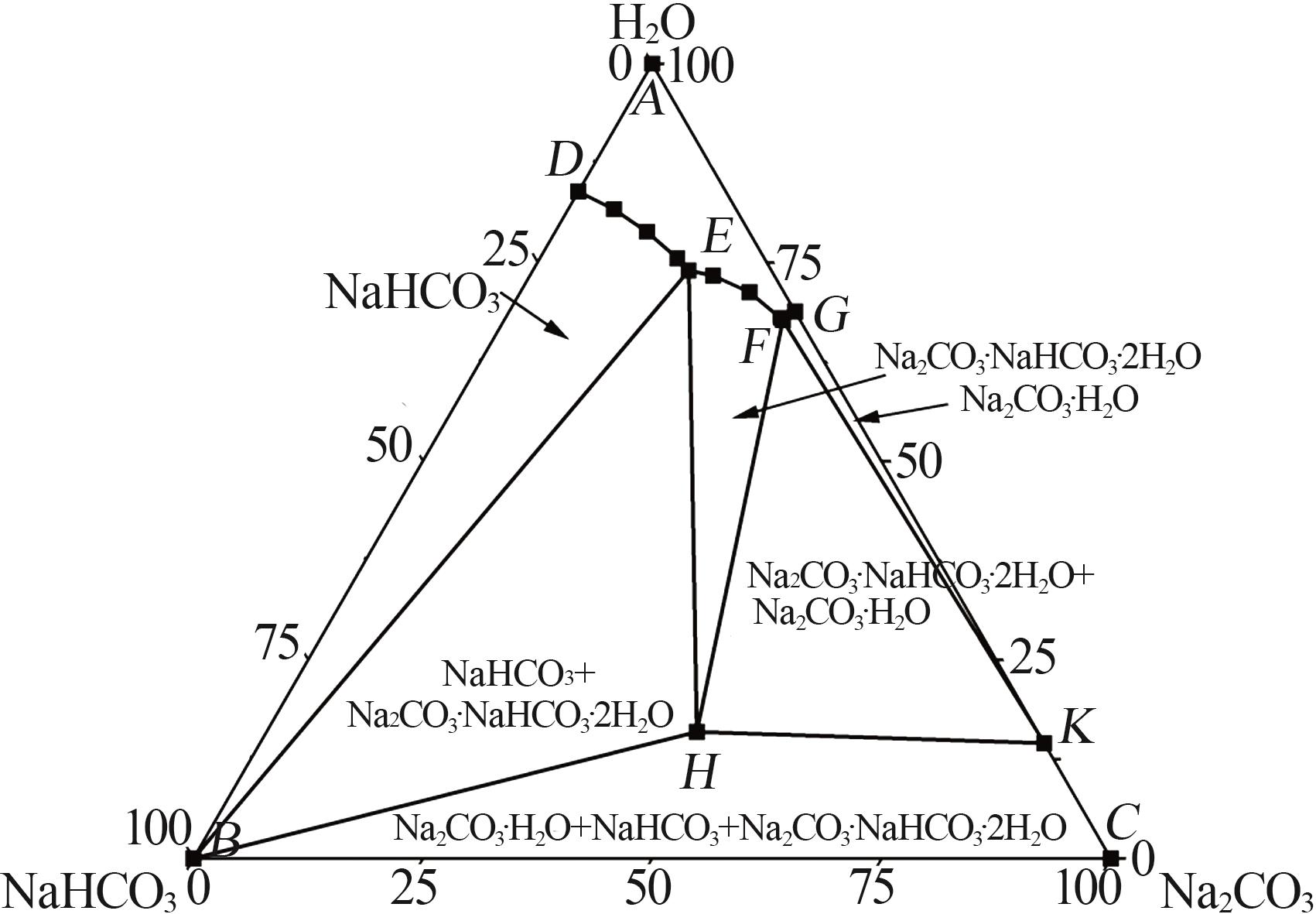
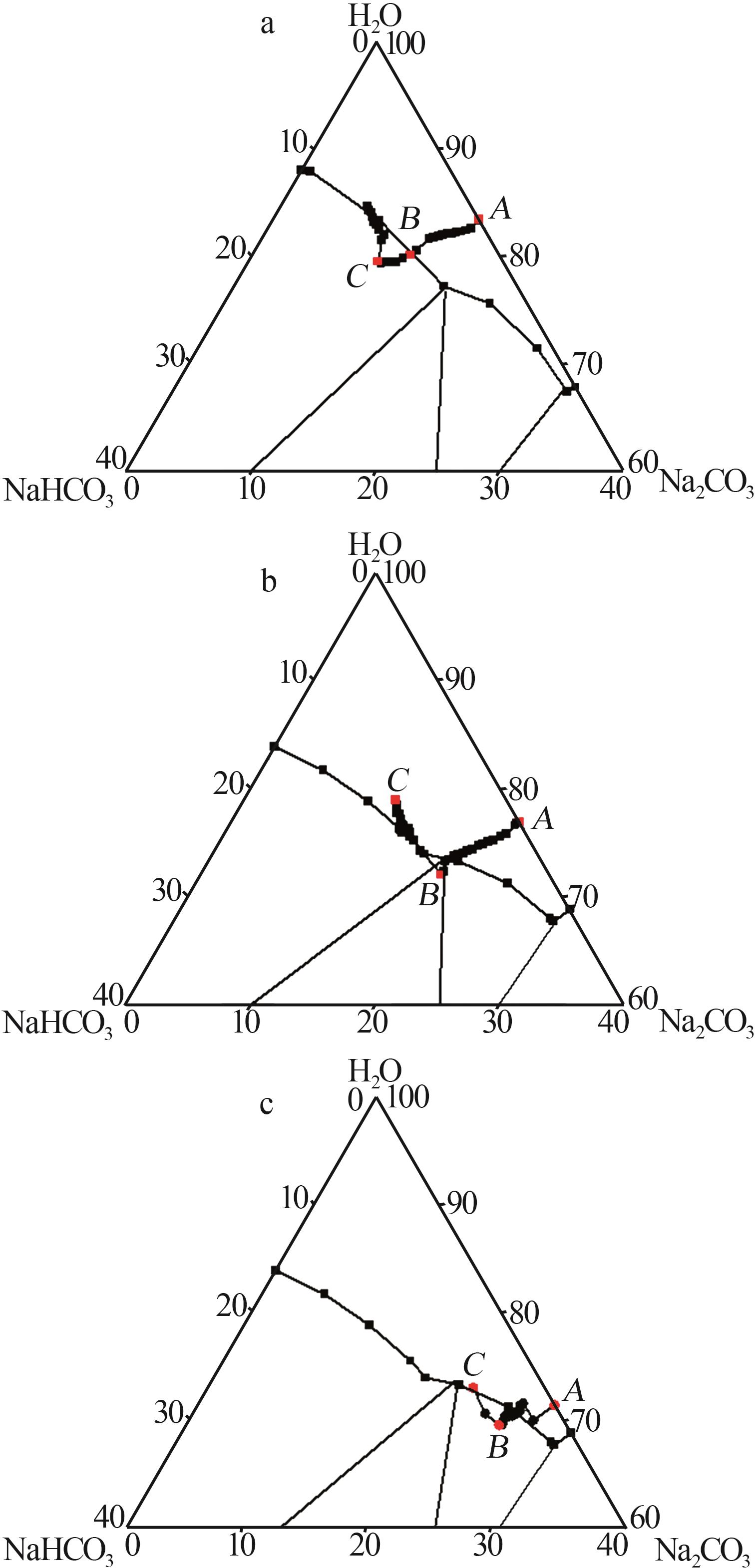
| [14] | 尹小春,丁珊修.生产倍半碳酸钠的相图分析[J].纯碱工业,2012(5):3-7. |
| YIN Xiaochun, DING Shanxiu.Phase diagram analysis of sodium sesquicarbonate production[J].Soda Industry,2012(5):3-7. | |
| [15] | KUDRYASHOVA O S, ELOKHOV A M, GARBUZ E E,et al.Phase equilibria in the K+,Na+||HCO3 -,HCOO–-H2O and K+,Na+||CO3 2-,HCOO–-H2O systems at 25 ℃[J].Russian Journal of Inorganic Chemistry,2020,65(12):1905-1912. |
| [16] | 邓天龙,周桓,陈侠.水盐体系相图及应用[M].2版.北京:化学工业出版社,2020. |
| [17] | 全煜丰.过程控制及分析技术在粒度分布和多晶型定量中的研究[D].天津:天津大学,2019. |
| QUAN Yufeng.Study on process control and analytical technology for shaping crystal size distribution and improving polymorphism quantification[D].Tianjin:Tianjin University,2019. | |
| [18] | 吴祥恩.含抑制剂体系甲烷水合物形成动力学的拉曼光谱研究[D].武汉:中国地质大学,2018. |
| WU Xiang′en. In-situ Raman spectroscopic study on the kinetics of methane hydrate formation in inhibitor containing system[D].Wuhan:China University of Geosciences,2018. | |
| [19] | 张艳平,薛冬峰.原位拉曼光谱技术用于反应和结晶控制研究进展[J].化学研究,2020,31(1):1-8. |
| ZHANG Yanping, XUE Dongfeng.Research progress of in situ Raman spectroscopy for chemical reaction and crystallization control[J].Chemical Research,2020,31(1):1-8. |
| [1] | LU Ziyu,LIU Zhaogang,WU Jinxiu,HU YanHong,LIU Xingyu. Study on phase equilibrium of ternary system of Na2CO3-NaF-H2O at 298.15 K and 318.15 K [J]. Inorganic Chemicals Industry, 2022, 54(3): 66-70. |
| [2] | Ye Weihui,Lü Qi,Long Changjiang. Study on rapid preparation process of high-density spherical basic nickel carbonate [J]. Inorganic Chemicals Industry, 2021, 53(5): 69-72. |
| [3] | Hu Caixia,Hu Chenxin,Peng Jianlong,Zhong Xing. Preparation of porous spherical calcium carbonate with soluble starch as crystallization controller [J]. Inorganic Chemicals Industry, 2021, 53(5): 51-55. |
| [4] | Ma Yuming. Synthesis and application of sodium carbonate solution crystallization inhibitor [J]. Inorganic Chemicals Industry, 2020, 52(8): 63-65. |
| [5] | Cheng Chunchun,Song Haoyu,Li Chunli. Analysis of material flow and energy flow of soda ash based on MFA [J]. Inorganic Chemicals Industry, 2020, 52(6): 59-62. |
| [6] | Hou Junwei,Hui Zeyou,Chen Zijing,Song Qi,Huang Bingxuan,Chen Mengmeng. Study on influencing factors of consumption of sodium carbonate and inorganic mineral in ASP flooding of conglomerate reservoirs [J]. Inorganic Chemicals Industry, 2020, 52(11): 16-19. |
| [7] | Fei Zhongxin,Zhu Liang,Zhao Jingxin,Wang Yanfei,Yang Libin,Sha Zuoliang,Zhao Xiaoyu. Investigation on removal process of sodium carbonate from methionine saponification solution using cooling crystallization [J]. Inorganic Chemicals Industry, 2019, 51(9): 81-84. |
| [8] | CHEN Shi-Hao. Production process of sodium metabisulfite with sodium carbonate decahydrate [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(6): 42-. |
| [9] | YANG Zhao-Juan, WANG Shun-Qiang, ZHAO Yan-Long, WANG Ji-Yang. Research on preparation process of sodium carbonate monohydrate from sodium carbonate decahydrate tailings [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(4): 56-. |
| [10] | WU Yong-Yong, ZHOU Yong-Min, ZHANG Su-Yi. Study on process parameters for activating fly ash with alkali by calcination [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(9): 45-. |
| [11] | CAI Qian, ZHOU Jun-Bo. Application of ion chromatography in determination of chloridion in separation of alkali and salt [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(6): 49-. |
| [12] | SU Xiu-Xia, JIANG Ji-Lei, LI Zhong-Jin, HUI Yuan-Yuan. Effect of sodium silicate and sodium carbonate on stability of sodium hypochlorite solution [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(11): 22-. |
| [13] | Zhou Guoe;Zhong Hongmei. New production technology for soda ash with salt slag by-produced from hydrazine hydrate production [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(8): 0-0. |
| [14] | Tang Kai;Yan Jie;Peng Chuanfeng;Lu Shan;Gao Hanyun. Extraction technology of lithium salt from waste catalysts [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(8): 0-0. |
| [15] | Guo Changming;Li Xuanhai;Liu Lifen;Sun Pengbo. Effect factors of decolorization in singlestep decolorization and defluorinate technology of wetprocess phosphoric acid [J]. INORGANICCHEMICALSINDUSTRY, 2009, 0(2): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||