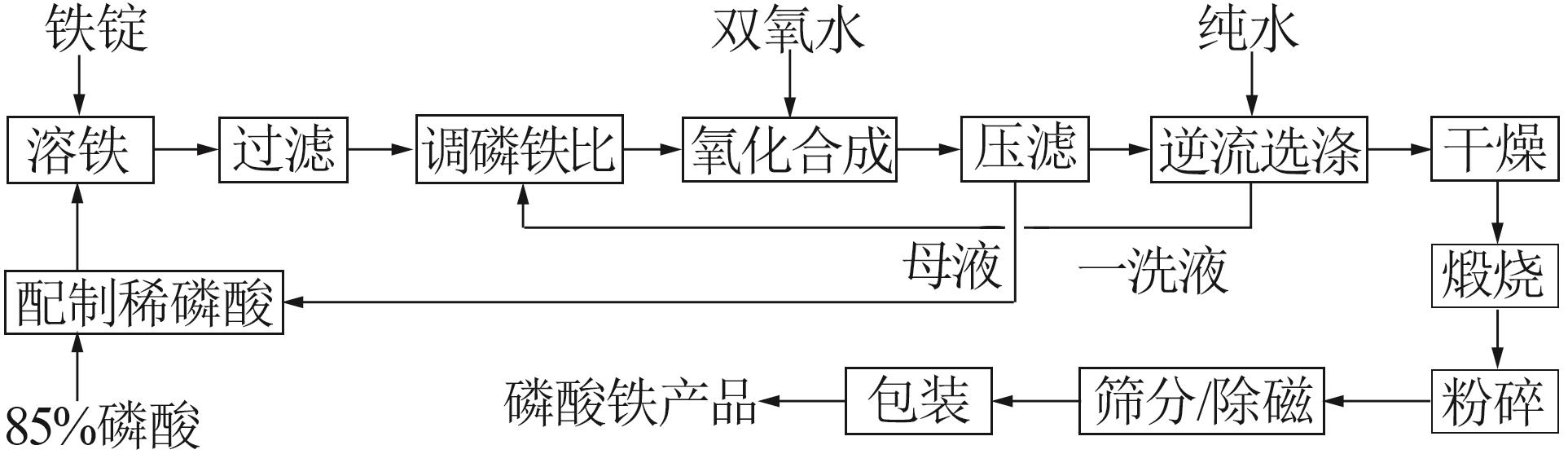
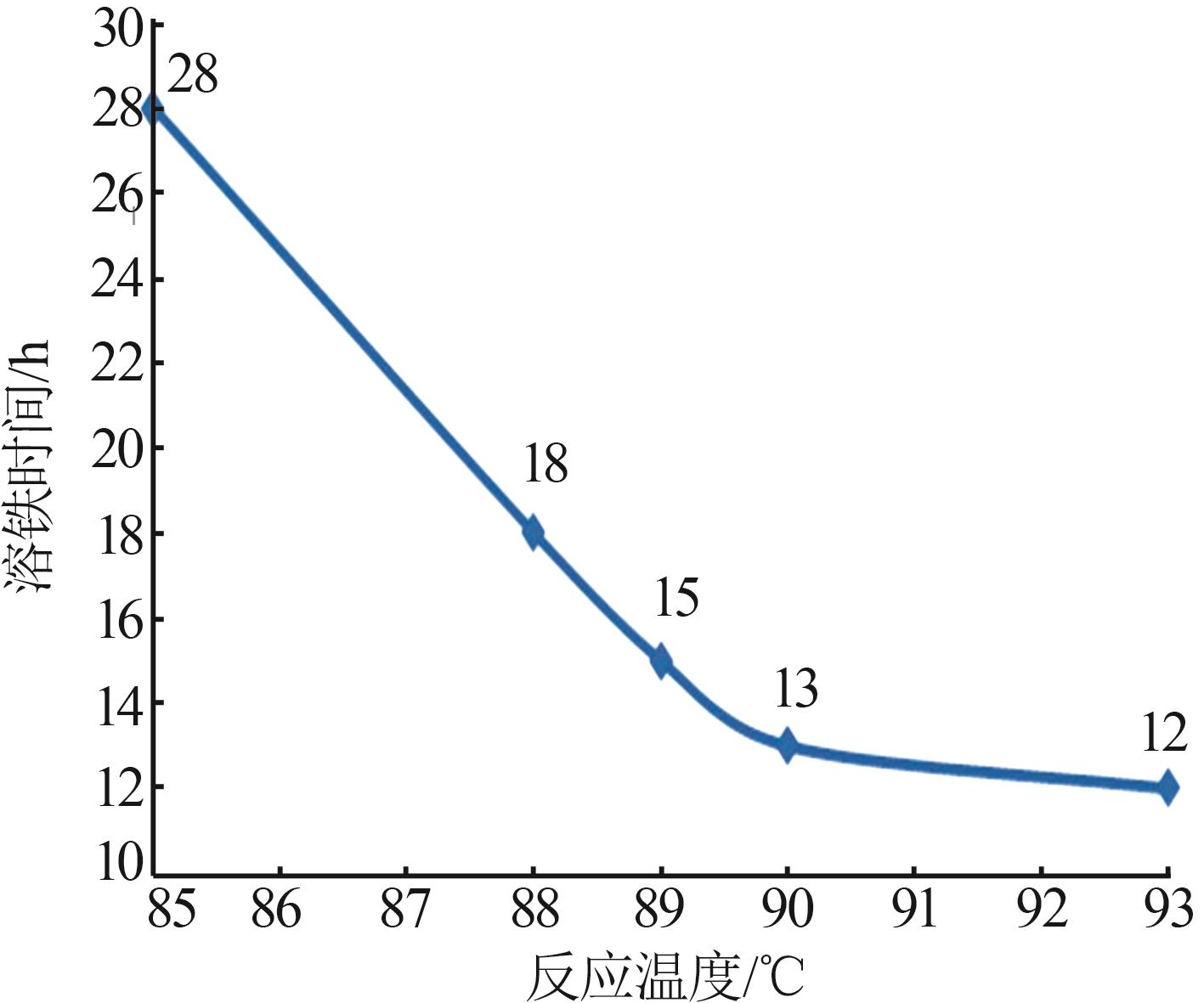
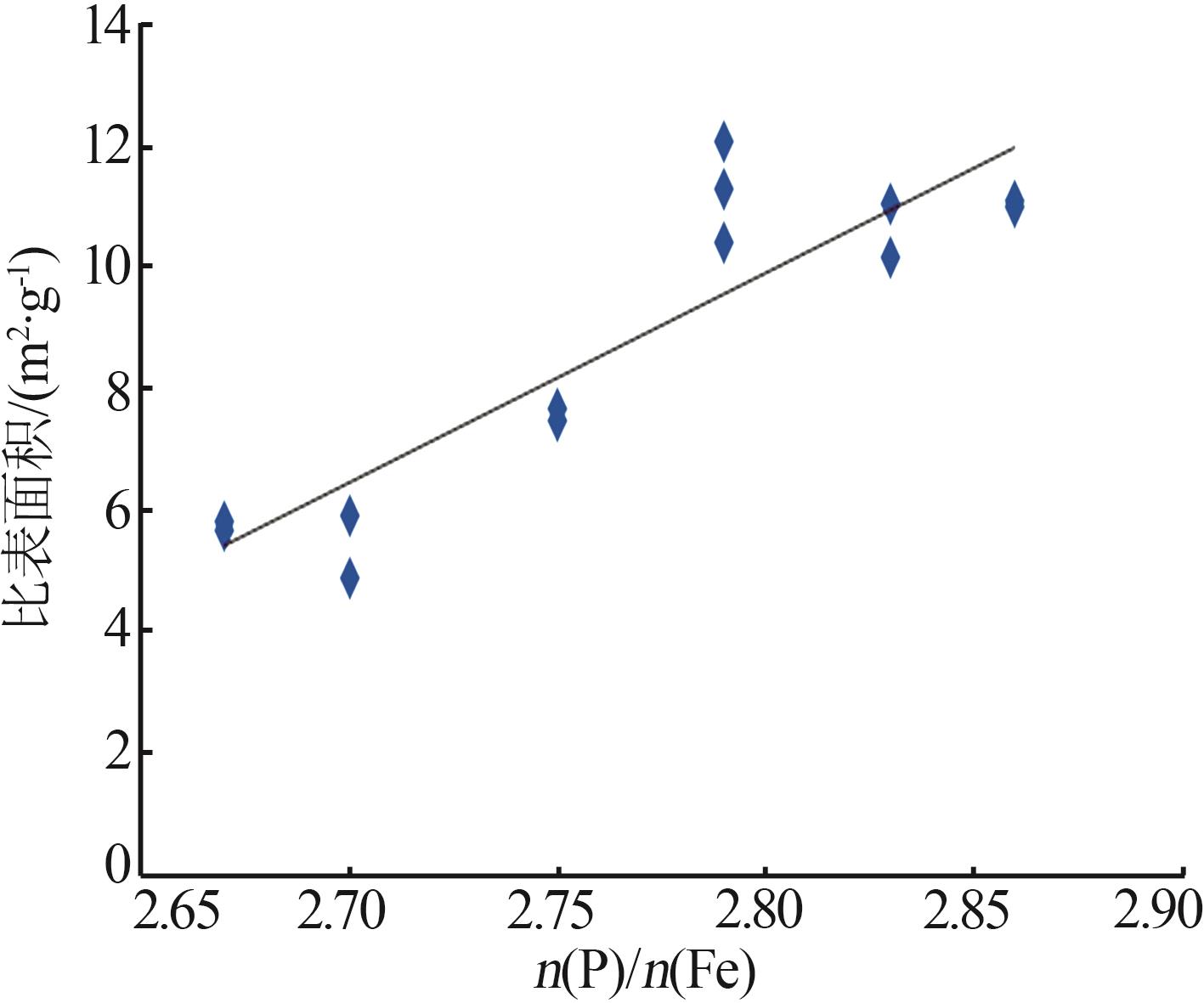
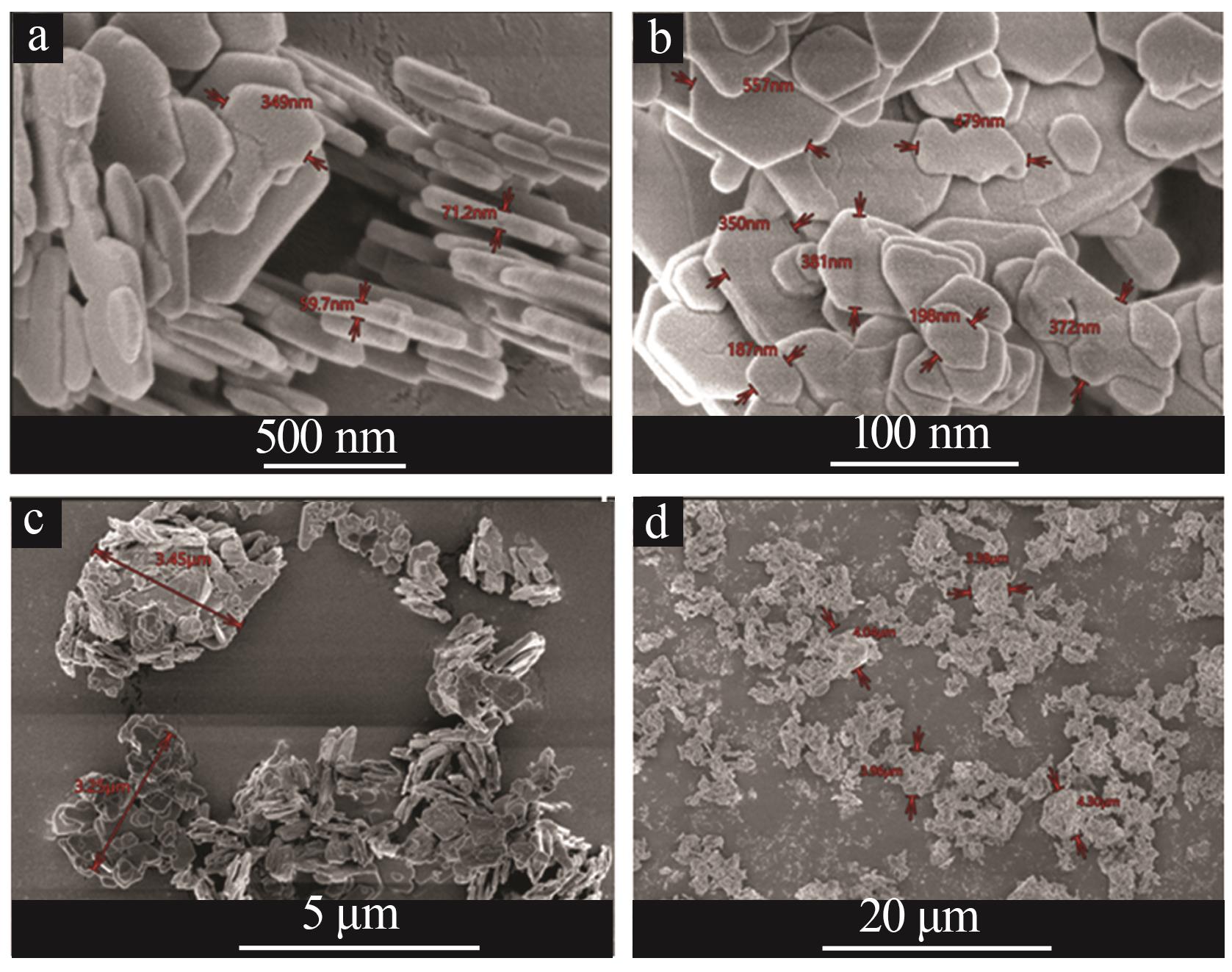
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (9): 73-81.doi: 10.19964/j.issn.1006-4990.2024-0450
• Research & Development • Previous Articles Next Articles
Study on iron-based process for preparing battery-grade ferric phosphate
NI Shuanglin1( ), MA Hang2(
), MA Hang2( ), XUE He′nan2, WAN Banglong2, YAN Yinxian2, ZHANG Jinyuan2, CHEN Zhanghong2, WEI Xing2, CHEN Yunjian1, DU Jianbo2, DAI Jinfeng2
), XUE He′nan2, WAN Banglong2, YAN Yinxian2, ZHANG Jinyuan2, CHEN Zhanghong2, WEI Xing2, CHEN Yunjian1, DU Jianbo2, DAI Jinfeng2
- 1. Yunnan Yuntianhua Phosphorus Industry Research Technology Co. ,Ltd. ,Kunming 650228,China
2. Yunnan Yuntianhua Co. ,Ltd. ,R&D Center,Kunming 650228,China
-
Received:2024-08-14Online:2025-09-10Published:2025-09-23 -
Contact:MA Hang E-mail:Shuanglin.ni@yprtec.com;hang.ma@yprtec.com
CLC Number:
Cite this article
NI Shuanglin, MA Hang, XUE He′nan, WAN Banglong, YAN Yinxian, ZHANG Jinyuan, CHEN Zhanghong, WEI Xing, CHEN Yunjian, DU Jianbo, DAI Jinfeng. Study on iron-based process for preparing battery-grade ferric phosphate[J]. Inorganic Chemicals Industry, 2025, 57(9): 73-81.
share this article
Table 1
Quality indicators of iron phosphate products"
| 指标项 | w(Fe)/ % | w(P)/ % | n(Fe)/n(P) | 振实密度/ (g·cm-3) | 比表面积/ (m2·g-1) | 粒度 (D50)/μm | w(磁性 物)/% | w(H2O)/ % | w(Ca)/ % | w(Mg)/ % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HG/T 4701—2021(Ⅰ) | 35.7~36.7 | 20.0~21.1 | 0.96~1.0 | ≥0.6 | 3~16 | 1~9 | ≤0.000 25 | ≤0.5 | ≤0.01 | ≤0.06 | ||||
| 自制(铁法工艺) | 36.18~36.58 | 20.40~20.70 | 0.970~0.985 | 0.60~0.85 | 6~9 | 3~5 | ≤0.000 05 | ≤0.24 | ≤0.002 7 | ≤0.000 2 | ||||
| 铵法两步法工艺 | 36.32 | 20.93 | 0.962 | 0.82 | 8.71 | 4.57 | ≤0.000 02 | ≤0.33 | ≤0.001 6 | ≤0.001 2 | ||||
| 指标项 | w(Na)/ % | w(K)/ % | w(Cu)/ % | w(Mn)/ % | w(Al)/ % | w(Ti)/ % | w(Co)/ % | w(Pb)/ % | w(Cr)/ % | w(S)/ % | ||||
| HG/T 4701—2021(Ⅰ) | ≤0.02 | ≤0.02 | ≤0.003 | ≤0.1 | ≤0.05 | ≤0.18 | — | — | — | ≤0.03 | ||||
| 自制(铁法工艺) | ≤0.001 1 | ≤0.000 98 | ≤0.000 4 | ≤0.001 2 | ≤0.005 6 | ≤0.000 05 | ≤0.000 19 | ≤0.000 89 | ≤0.002 6 | ≤0.000 3 | ||||
| 铵法两步法工艺 | ≤0.000 5 | ≤0.000 39 | ≤0.000 2 | ≤0.002 5 | ≤0.000 3 | ≤0.000 26 | ≤0.000 14 | ≤0.000 86 | ≤0.000 9 | ≤0.004 6 | ||||
| [1] | OKADA S, YAMAMOTO T, OKAZAKI Y,et al.Cathode properties of amorphous and crystalline FePO4 [J].Journal of Power Sources,2005,146(1/2):570-574. |
| [2] | 袁文龙,王碧侠,赵瑛,等.用钛白副产硫酸亚铁合成磷酸铁前驱体[J].有色金属工程,2023,13(7):61-68. |
| YUAN Wenlong, WANG Bixia, ZHAO Ying,et al.Synthesis of iron phosphate precursor from by-product ferrous sulfate of titanium dioxide[J].Nonferrous Metals Engineering,2023,13(7):61-68. | |
| [3] | 王紫涵,李军,陈明,等.硝酸铁和磷酸共沉淀法制备电池级磷酸铁工艺研究[J].无机盐工业,2023,55(7):51-57. |
| WANG Zihan, LI Jun, CHEN Ming,et al.Study on preparation of battery grade ferric phosphate by co-precipitation of ferric nitrate and phosphoric acid[J].Inorganic Chemicals Industry,2023,55(7):51-57. | |
| [4] | 李加勇,白志鹏,郑清清.一种制备锂电池用LiFePO4的方法[J].电源技术,2023,47(8):1002-1005. |
| LI Jiayong, BAI Zhipeng, ZHENG Qingqing.A method for synthetizing LiFePO4 used in lithium ion batteries[J].Chinese Journal of Power Sources,2023,47(8):1002-1005. | |
| [5] | 刘万丰,肖仁贵,林倩,等.磷铁制备电池级磷酸铁放大实验研究[J].无机盐工业,2015,47(5):75-78. |
| LIU Wanfeng, XIAO Rengui, LIN Qian,et al.Amplification experiments of preparing battery-grade ferric phosphate by ferrophosphorus[J].Inorganic Chemicals Industry,2015,47(5):75-78. | |
| [6] | 苏勇杰,张勇,陈喆,等.圆片状超细二水磷酸铁的制备与表征[J].武汉工程大学学报,2018,40(1):66-70. |
| SU Yongjie, ZHANG Yong, CHEN Zhe,et al.Preparation and char-acterization of ultrafine disc shaped ferric phosphate dihydrate[J].Journal of Wuhan Institute of Technology,2018,40(1):66-70. | |
| [7] | 李永佳,魏润宏,鲁劲华,等.电池级磷酸铁的制备及性能[J].化工进展,2021,40(4):2227-2233. |
| LI Yongjia, WEI Runhong, LU Jinhua,et al.Preparation and performance of FePO4 precursor for LiFePO4 [J].Chemical Industry and Engineering Progress,2021,40(4):2227-2233. | |
| [8] | 闫银贤,马航,万邦隆,等.铁法磷酸铁的工业化制备及其电化学应用[J].云南化工,2024,51(2):80-83. |
| YAN Yinxian, MA Hang, WAN Banglong,et al.Industrial preparation and electrochemical application of iron phosphate by pure iron method[J].Yunnan Chemical Technology,2024,51(2):80-83. | |
| [9] | 宋依桐,李成威,刘钢,等.水雾化铁粉制备微纳米磷酸铁的实验研究[J].辽宁科技大学学报,2020,43(4):241-244,250. |
| SONG Yitong, LI Chengwei, LIU Gang,et al.Experimental study on preparation of micro-nano iron phosphate with water-atomized iron powder[J].Journal of University of Science and Technology Liaoning,2020,43(4):241-244,250. | |
| [10] | 王红强,柯君雄,王镖,等.以冷轧板为铁源制备片状磷酸铁[J].化工管理,2023(22):143-146. |
| WANG Hongqiang, KE Junxiong, WANG Biao,et al.Preparation of flaky shaped FePO4 with cold rolled plate as iron source[J].Chemical Engineering Management,2023(22):143-146. | |
| [11] | 马航,倪双林,万邦隆,等.一种连续氧化工艺制备磷酸铁的方法:中国,116534824A[P].2023-08-04. |
| [12] | 苏勇杰.以还原铁粉为铁源制备磷酸铁的基础研究[D].武汉:武汉工程大学,2019. |
| SU Yongjie.Basic research on Preparation of ferric phosphate from reduced iron powder as iron source[D].Wuhan:Wuhan Institute of Technology,2019. | |
| [13] | TAKAHASHI M, TOBISHIMA S, TAKEI K,et al.Characterization of LiFePO4 as the cathode material for rechargeable lithium batteries[J].Journal of Power Sources,2001,97:508-511. |
| [1] | CHEN Mengmeng, XU Dekan, HUANG Jilong, TANG Zhilan, ZHANG Xu, TAN Chao, WANG Xiaohu, PENG Wenbo. Study on preparation and performance of Ni-modified titanium-based lithium ion sieve [J]. Inorganic Chemicals Industry, 2025, 57(9): 37-45. |
| [2] | DANG Jianmeng, ZHANG Zhichao, CAO Zhongkai, LI Zixuan, SHEN Jixue. Research on improving electrochemical performance of Ni-rich cathode materials under fast-charging operation [J]. Inorganic Chemicals Industry, 2025, 57(7): 14-23. |
| [3] | XU Xiaojing, ZHANG Yuanyuan, LIU Wenqian, LIU Shuyan, WANG Fei, GAO Yangyan, YANG Yuhua, YANG Fengling. Effect of liquid phase impurity Fe and Al ions on crystallization of ammonium sulfate in ammonia flue gas desulphurization [J]. Inorganic Chemicals Industry, 2025, 57(6): 27-34. |
| [4] | REN Genkuan, LUO Xin, ZHU Denglei, ZHANG Min. Thermodynamic analysis and experiment research on preparation of α-Fe2O3nanoparticles by solid-phase reduction method [J]. Inorganic Chemicals Industry, 2025, 57(4): 73-78. |
| [5] | YU Hesong, WANG Dexi, YU Honglei, ZUO Maosheng, LI Jiazhi. Preparation of battery-grade iron phosphate by jet-enhanced air oxidation method [J]. Inorganic Chemicals Industry, 2025, 57(4): 31-36. |
| [6] | WANG You, LIAO Lianzhen, CHEN Zheng, GAO Youjun. Effect of surfactants on electrocrystallization of Ni(OH)2 [J]. Inorganic Chemicals Industry, 2025, 57(3): 58-63. |
| [7] | LI Jiexuan, JIN Huiming. Study on preparation of Co-P-B/ZIF-67 catalyst and catalyzing hydrogen production from sodium borohydride hydrolysis [J]. Inorganic Chemicals Industry, 2024, 56(12): 150-158. |
| [8] | LI Ping, LI Jun, CHEN Ming. Study on process of recovery of Fe(Ⅲ) from spent lithium extractant and preparation of battery grade iron phosphate [J]. Inorganic Chemicals Industry, 2024, 56(10): 28-37. |
| [9] | WANG Junting, MA Hang, ZHA Zuotong, WAN Banglong, ZHANG Zhenhuan. Research progress of iron phosphate industrial wastewater treatment process [J]. Inorganic Chemicals Industry, 2024, 56(6): 26-33. |
| [10] | CHEN Yuneng, CHEN Kunfeng, XUE Dongfeng. Research progress of preparation and device application of lithium niobate crystal ferroelectric domain [J]. Inorganic Chemicals Industry, 2024, 56(6): 1-13. |
| [11] | LIU Hui, WANG Hongliang, YU Kun, GAO Shengnan. Effect of calcination on porous structure and electrochemical properties of air electrode [J]. Inorganic Chemicals Industry, 2024, 56(6): 80-86. |
| [12] | LI Kuai, LI Zhaoshuai, DONG Tingxuan, LI Dan, GUO Shengwei, HAN Fenglan. Study on effect of wet magnetic separation on distribution of Fe and heavy metal in fly ash [J]. Inorganic Chemicals Industry, 2024, 56(4): 98-104. |
| [13] | LIU Xiao, XIE Lei, DENG Jifeng, SHI Yu, ZHENG Jie. Study on performance of sponge supported Co-B catalyst for hydrogen generation from catalytic hydrolysis of sodium borohydride [J]. Inorganic Chemicals Industry, 2023, 55(12): 146-151. |
| [14] | WANG Zihan, LI Jun, CHEN Ming, ZHOU Qingyu. Study on preparation of battery grade ferric phosphate by co-precipitation of ferric nitrate and phosphoric acid [J]. Inorganic Chemicals Industry, 2023, 55(7): 51-57. |
| [15] | XU Li, ZHANG Qiang. Experimental study on properties of iron tailings powder cement-based materials [J]. Inorganic Chemicals Industry, 2023, 55(6): 116-123. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||