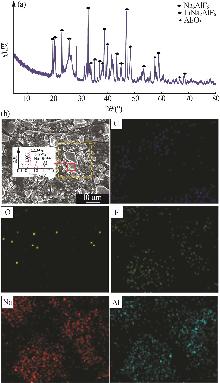
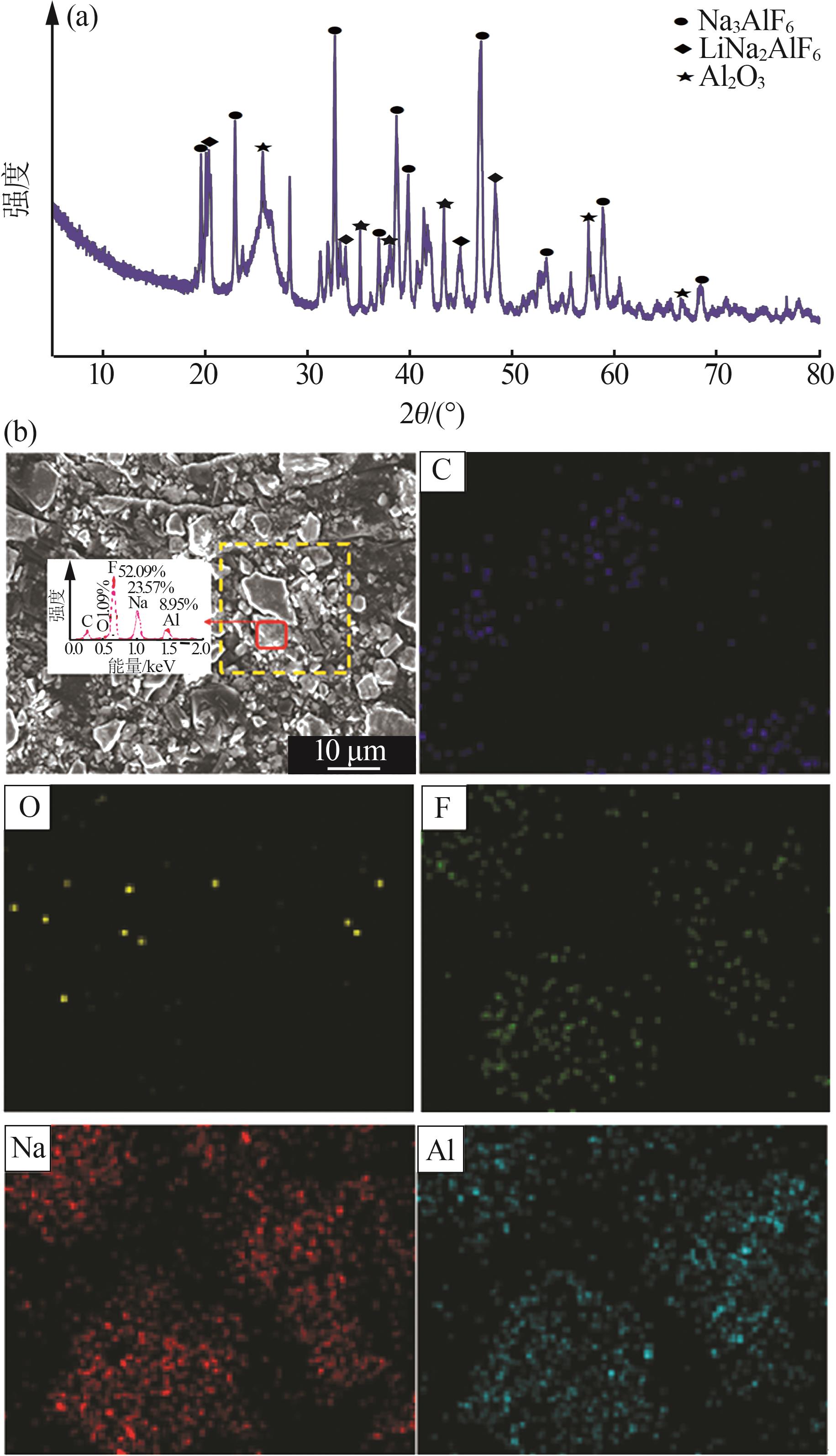
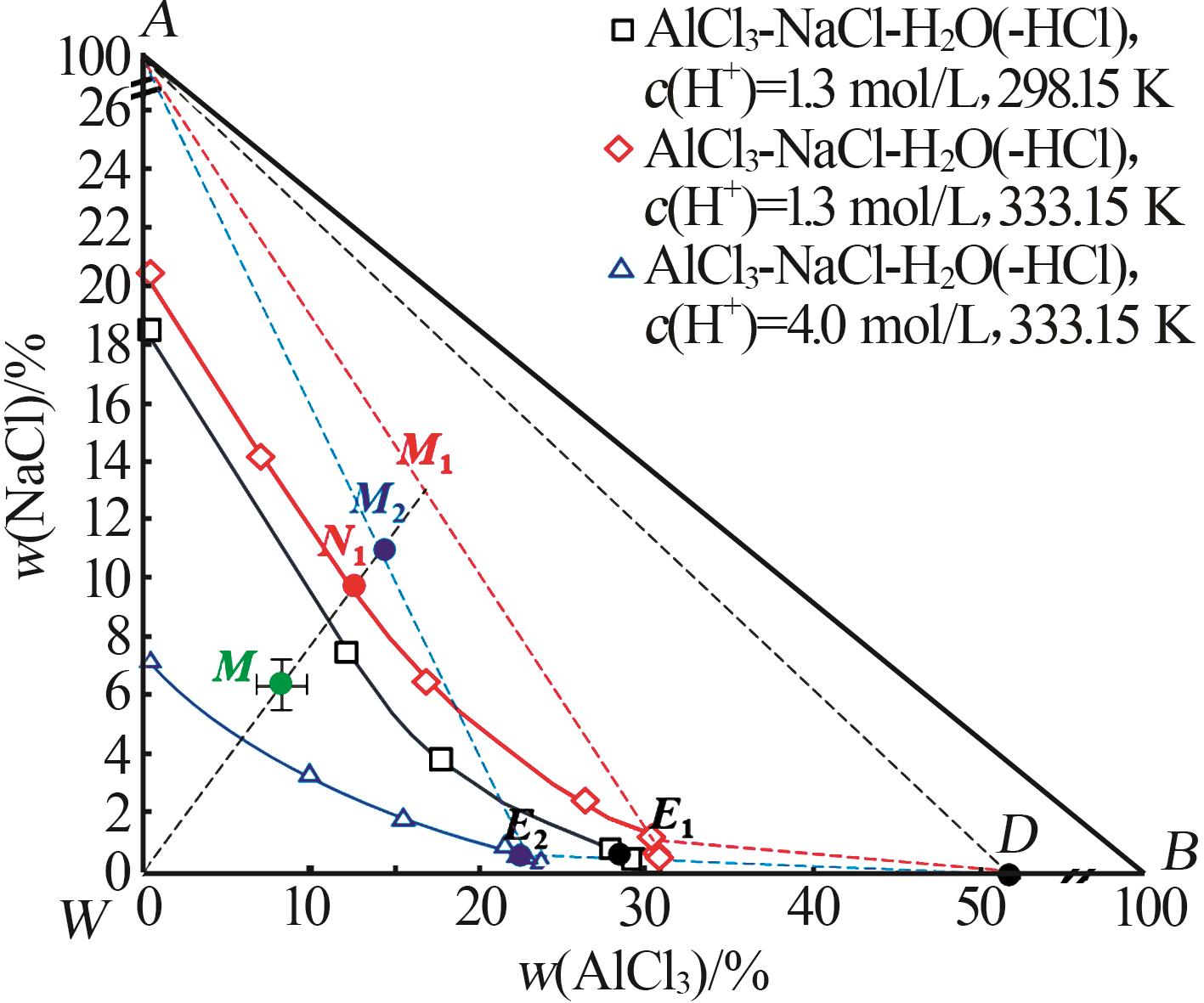
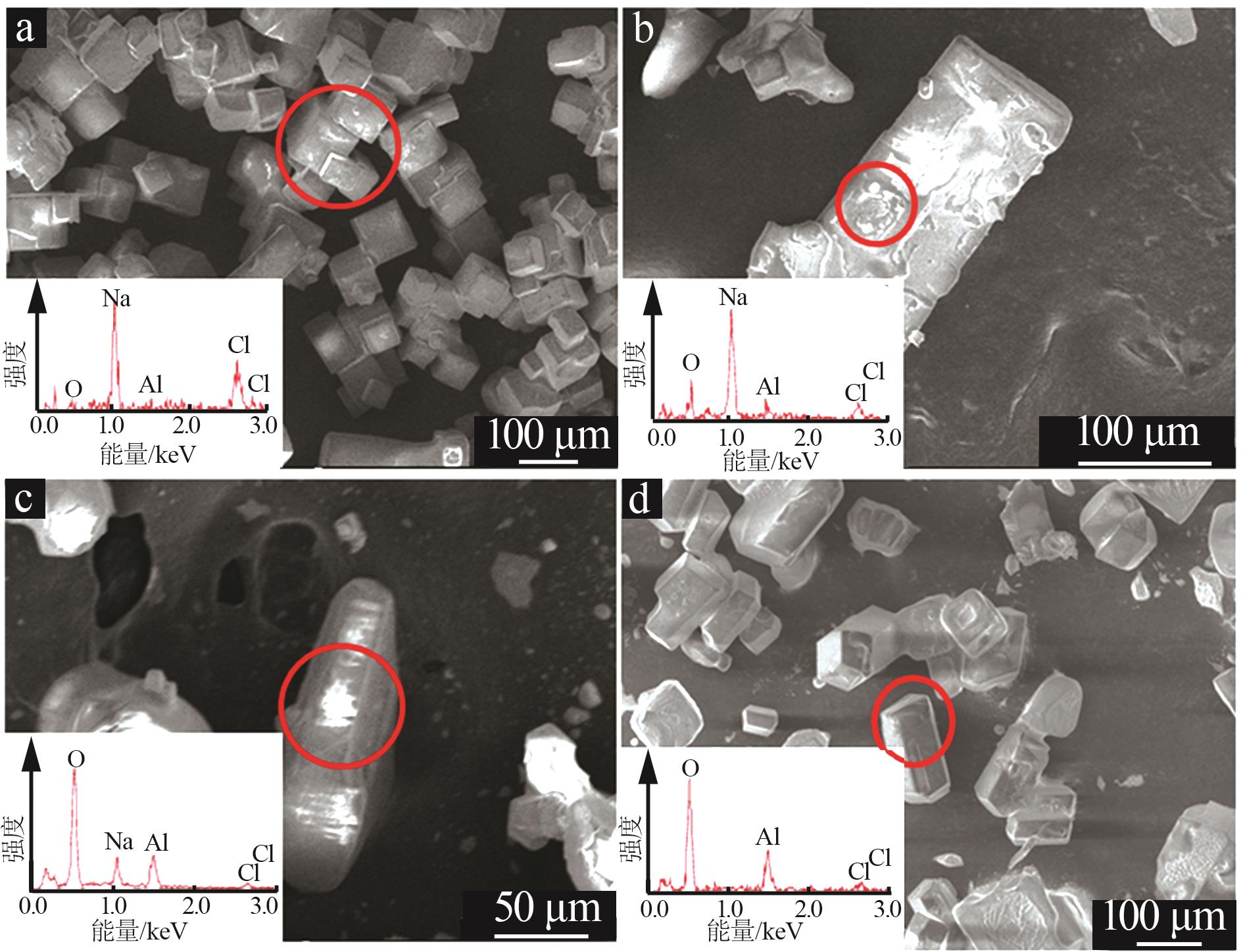
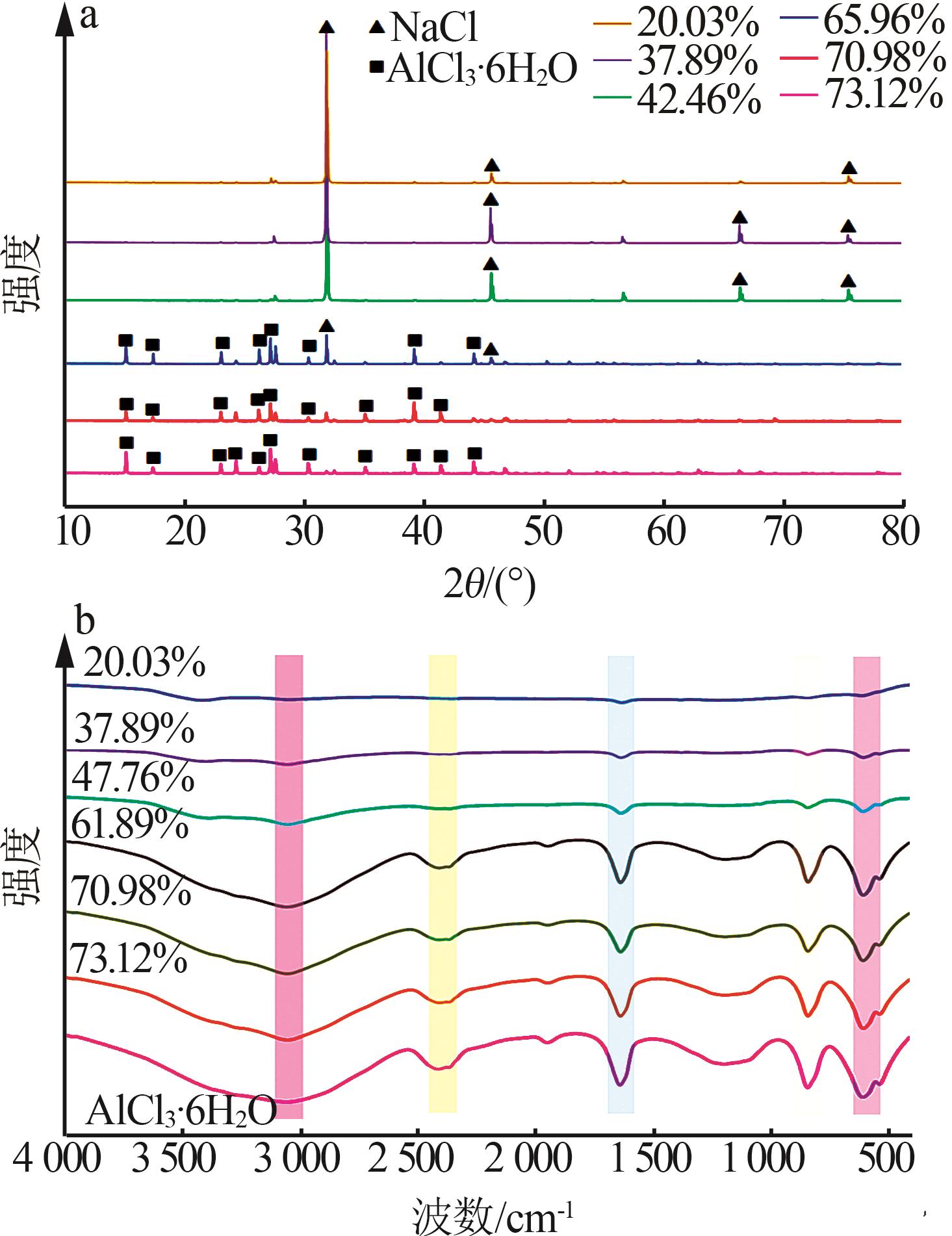
Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (5): 100-107.doi: 10.19964/j.issn.1006-4990.2024-0411
• Environment·Health·Safety • Previous Articles Next Articles
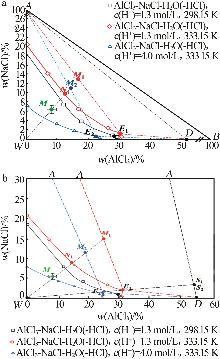
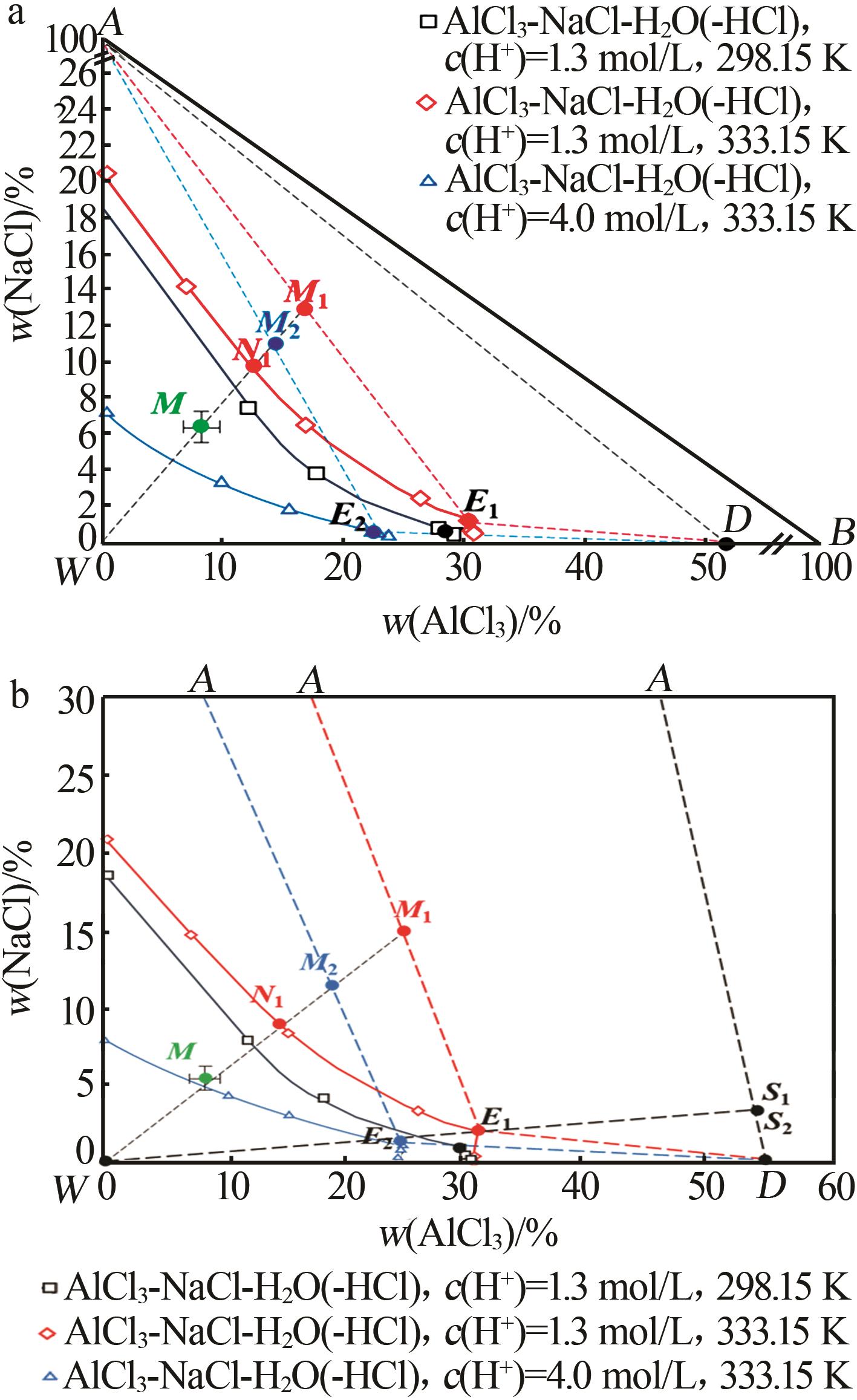
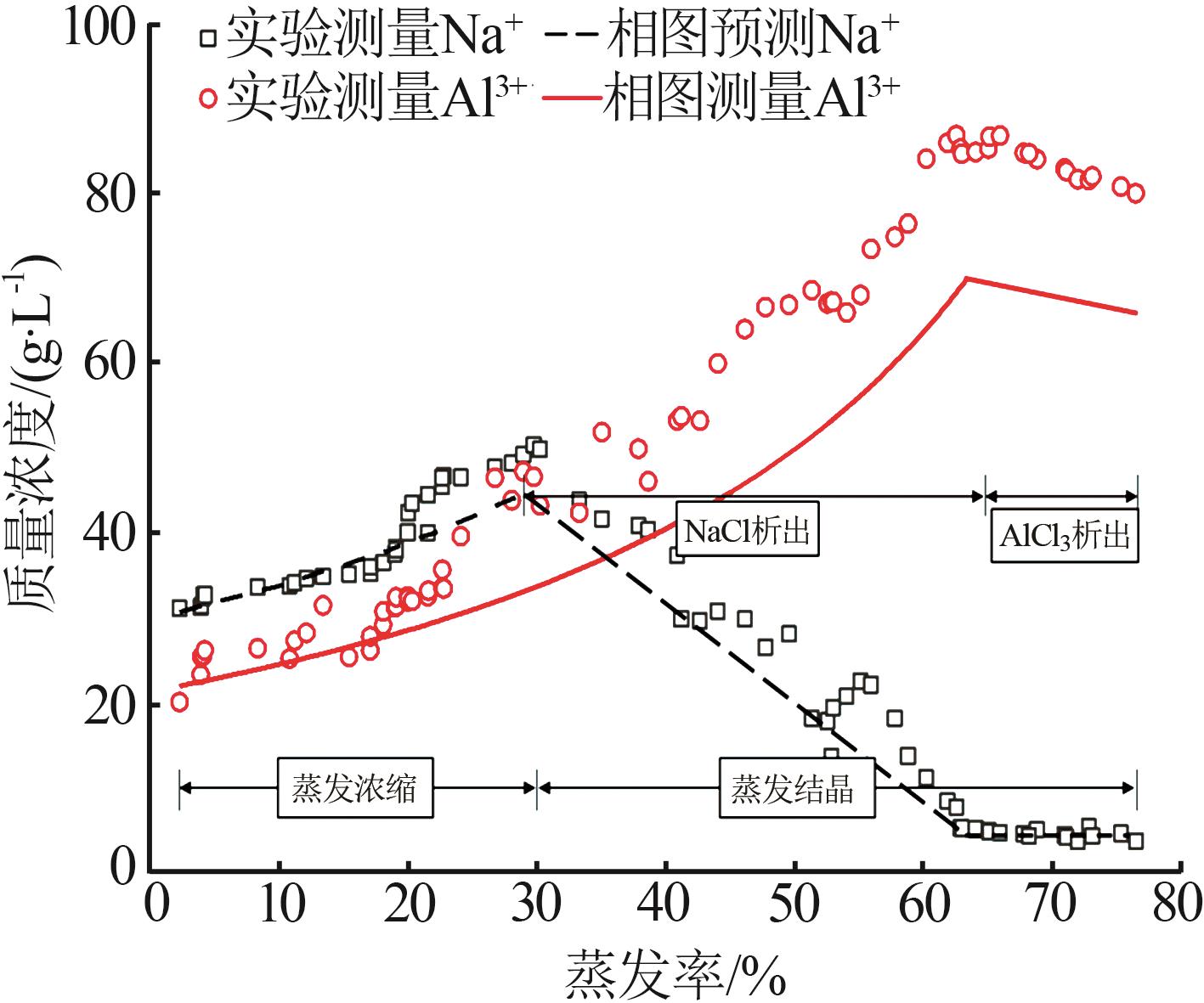
Study on crystallization and separation process of high⁃purity AlCl3·6H2O from acid leach solution of electrolytic aluminum scrap
SUN Liping1( ), CHENG Huaigang1,2(
), CHENG Huaigang1,2( ), GUO Wenxuan1, CUI Li1
), GUO Wenxuan1, CUI Li1
- 1. Institute of Resource and Environmental Engineering,Shanxi University,Shanxi Yellow River Laboratory,Taiyuan 030032,China
2. College of Chemical Engineering,Qinghai University,Xining 810016,China
-
Received:2024-07-18Online:2025-05-10Published:2025-06-05 -
Contact:CHENG Huaigang E-mail:313237930@qq.com;chenghg@sxu.edu.cn
CLC Number:
Cite this article
SUN Liping, CHENG Huaigang, GUO Wenxuan, CUI Li. Study on crystallization and separation process of high⁃purity AlCl3·6H2O from acid leach solution of electrolytic aluminum scrap[J]. Inorganic Chemicals Industry, 2025, 57(5): 100-107.
share this article
| 1 | ZHAO Qiuping, WANG Yiru, DONG Hong,et al.Preparation of anode materials for lithium⁃ion batteries by spent carbon anode from electrolytic aluminum[J].Journal of Environmental Chemical Engineering,2021,9(5):105932. |
| 2 | YUAN Jie, XIAO Jin, TIAN Zhongliang,et al.Optimization of purification treatment of spent cathode carbon from aluminum electrolysis using response surface methodology(RSM)[J].Asia⁃Pacific Journal of Chemical Engineering,2018,13(1):e2164. |
| 3 | VASYUNINA N V, BELOUSOV S V, DUBOVA I V,et al.Recovery of silicon and iron oxides from alumina⁃containing sweepings of aluminum production[J].Russian Journal of Non⁃Ferrous Metals,2018,59(3):230-236. |
| 4 | LI Huateng, WU Pan, ZHAO Guowei,et al.Fabrication of industrial⁃level polymer photonic crystal films at ambient temperature based on uniform core/shell colloidal particles[J].Journal of Colloid and Interface Science,2021,584:145-153. |
| 5 | KOLESNIKOV V A, IL’IN V I, BRODSKIY V A,et al.Electroflotation during wastewater treatment and extraction of valuable compounds from liquid technogenic waste:A review[J].Theoretical Foundations of Chemical Engineering,2017,51(4):369-383. |
| 6 | YUAN Ya, YU Xiaohua, SHEN Qingfeng,et al.A novel approach for ultrasonic assisted organic acid leaching of waste lithium⁃containing aluminum electrolyte and recovery of lithium[J].Chemical Engineering and Processing-Process Intensification,2023,192:109508. |
| 7 | LIANG Qian, WANG Jiqin, CHEN Shuyuan,et al.Electrolyte circulation:Metal recovery from waste printed circuit boards of mobile phones by alkaline slurry electrolysis[J].Journal of Cleaner Production,2023,409:137223. |
| 8 | YUAN Jie, DING Shuang, LI Huijin.Purification of spent cathode carbon from aluminum electrolysis cells by alkali fusion⁃acid leaching process[J].Journal of Material Cycles and Waste Management,2022,24(6):2608-2619. |
| 9 | 朱朝梁,温现明,邓小川,等.酸沉-萃取-结晶法从高镁卤水中提取硼酸的工艺研究[J].无机盐工业,2016,48(10):20-22. |
| ZHU Chaoliang, WEN Xianming, DENG Xiaochuan,et al.Research on extracting boric acid from high magnesium brines by combined process of acidification precipitation⁃centrifugal solvent extraction⁃dissolution crystallization[J].Inorganic Chemicals Industry,2016,48(10):20-22. | |
| 10 | 刘红,张一敏,黄晶.N235支撑液膜分离页岩提钒酸浸液及传质机理[J].中国有色金属学报,2020,30(9):2216-2223. |
| LIU Hong, ZHANG Yimin, HUANG Jing.Separation and transfer mechanism of vanadium from black shale leaching solution by supported liquid membrane using N235[J].The Chinese Journal of Nonferrous Metals,2020,30(9):2216-2223. | |
| 11 | 周为峰,池勇志,李恺雄,等.离子交换法脱除湿法冶金工业MVR冷凝废水中盐类的研究[J].无机盐工业,2022,54(4):152-158. |
| ZHOU Weifeng, CHI Yongzhi, LI Kaixiong,et al.Study on removal of salts from MVR condensate wastewater in the hydrometallurgical industry by ion exchange method[J].Inorganic Chemicals Industry,2022,54(4):152-158. | |
| 12 | 王松,王家伟,勾碧波,等.复盐结晶法对除菱锰矿浸出液中镁分配规律研究[J].无机盐工业,2023,55(4):65-71. |
| WANG Song, WANG Jiawei, GOU Bibo,et al.Study on distribution law of magnesium in leaching solution of rhodochrosite by complex salt crystallization method[J].Inorganic Chemicals Industry,2023,55(4):65-71. | |
| 13 | 成春春,李玉龙,张志强,等.溶析结晶用于盐湖卤水提钾和镁锂分离研究[J].无机盐工业,2024,56(6):34-39. |
| CHENG Chunchun, LI Yulong, ZHANG Zhiqiang,et al.Study on dissolution crystallization for extraction of potassium and separation of magnesium and lithium from salt lake brine[J].Inorganic Chemicals Industry,2024,56(6):34-39. | |
| 14 | 王斌,邓小川,史一飞,等.田口实验设计法优化碳酸锂反应结晶制备工艺[J].无机盐工业,2021,53(8):60-65. |
| WANG Bin, DENG Xiaochuan, SHI Yifei,et al.Optimizing preparation process of lithium carbonate reaction crystallization by Taguchi experimental design method[J].Inorganic Chemicals Industry,2021,53(8):60-65. | |
| 15 | 靳苏娜,吕瑞亮.湿法脱硫废水处理技术研究及应用进展[J].无机盐工业,2023,55(4):27-37. |
| JIN Suna, Ruiliang LÜ.Research and application progress of wet flue gas desulfurization wastewater treatment technology[J].Inorganic Chemicals Industry,2023,55(4):27-37. | |
| 16 | 吕慧斌.粉煤灰酸浸液中AlCl3·6H2O的结晶分离研究[D].太原:山西大学,2017. |
| Huibin LÜ.Crystallization separation of AlCl3·6H2O from acid leaching liquor of coal fly ash[D].Taiyuan:Shanxi University,2017. | |
| 17 | YUAN Mengxia, QIAO Xiuchen, Yu Jianguo.Phase equilibria of AlCl3+FeCl3+H2O,AlCl3+CaCl2+H2O,and FeCl3+CaCl2+H2O at 298.15 K[J].Journal of Chemical & Engineering Data,2016,61(5):1749-1755. |
| 18 | 袁梦霞,乔秀臣.三元体系AlCl3+CaCl2+H2O,AlCl3+FeCl3+H2O和CaCl2+FeCl3+H2O在35 ℃时的相平衡[J].化工学报,2017,68(7):2653-2659. |
| YUAN Mengxia, QIAO Xiuchen.Phase equilibria of AlCl3+CaCl2+H2O,AlCl3+FeCl3+H2O and CaCl2+FeCl3+H2O ternary systems at 35 ℃[J].CIESC Journal,2017,68(7):2653-2659. | |
| 19 | GAO Wencheng, LI Zhibao, ASSELIN E.Solubility of AlCl3·6H2O in the Fe(Ⅱ)+Mg+Ca+K+Cl+H2O system and its salting⁃out crystallization with FeCl2 [J].Industrial & Engineering Chemistry Research,2013,52(39):14282-14290. |
| 20 | CUI Li, FENG Lijuan, YUAN Hefeng,et al.Efficient recovery of aluminum,lithium,iron and gallium from coal fly ash leachate via coextraction and stepwise stripping[J].Resources,Conservation and Recycling,2024,202:107380. |
| 21 | CUI Li, WANG Weihong, CHAO Xi,et al.Efficient lithium recovery from electrolytic aluminum slag via an environmentally friendly process:Leaching behavior and mechanism[J].Journal of Cleaner Production,2024,439:140800. |
| 22 | WU Shaohua, TAO Wenju, ZHENG Yanchen,et al.Novel process for the extraction of lithium carbonate from spent lithium⁃containing aluminum electrolytes by leaching with aluminum nitrate and nitric acid[J].Hydrometallurgy,2020,198:105505. |
| 23 | 王丹琴,刘秀,练以诚,等.一种电解铝大修渣资源化处理方法:中国,117735578A[P].2024-03-22. |
| 24 | 陶文举,陈煜东,杨佳鑫,等.一种回收铝电解质中铝和氟元素的方法:中国,117963846A[P].2024-05-03. |
| 25 | 郭彦霞,杨喜,崔慧霞,等.AlCl3·6H2O在盐酸体系中的结晶行为[J].化工学报,2014,65(10):3960-3967. |
| GUO Yanxia, YANG Xi, CUI Huixia,et al.Crystallization behavior of AlCl3·6H2O in hydrochloric system[J].CIESC Journal,2014,65(10):3960-3967. | |
| 26 | GUO Yanxia, LV Huibin, YANG Xi,et al.AlCl3⋅6H2O recovery from the acid leaching liquor of coal gangue by using concentrated hydrochloric inpouring[J].Separation and Purification Technology,2015,151:177-183. |
| 27 | 成怀刚,程芳琴.水盐体系相分离[M].北京:冶金工业出版社,2022:149-154. |
| 28 | 崔慧霞,程文婷,郭彦霞,等.六水合氯化铝在不同氯化物体系中的溶解度现象研究[J].无机盐工业,2013,45(5):9-12. |
| CUI Huixia, CHENG Wenting, GUO Yanxia,et al.Study on solubility phenomena of AlCl3·6H2O in different chloride systems[J].Inorganic Chemicals Industry,2013,45(5):9-12. | |
| 29 | FARELO F, FERNANDES C, AVELINO A.Solubilities for six ternary systems:NaCl+NH4Cl+H2O, KCl+NH4Cl+H2O, NaCl+LiCl+H2O, KCl+LiCl+H2O, NaCl+AlCl3+H2O, and KCl+AlCl3+H2O at T=(298 to 333) K[J]. Journal of Chemical & Engineering Data,2005, 50(4):1470-1477. |
| 30 | SHANKS D E, EISELE J A, BAUER D J.Hydrogen chloride sparging crystallization of aluminum chloride hexahydrate[M].Washington,D.C.:US Department of the Interior,Bureau of Mines,1981. |
| [1] | YU Xudong, LI Jing, REN Siying, LUO Jun, ZENG Ying. Study on solid-liquid phase equilibrium of Li+,K+,Ca2+//Cl--H2O quaternary system at 298.2 K [J]. Inorganic Chemicals Industry, 2025, 57(3): 30-35. |
| [2] | YANG Yousheng, YAO Zhihao, ZHAO Zhixing, FENG Xia, ZENG Ying, YU Xudong. Research progress of lithium-rich sulfate type salt lake brine evaporation experiment [J]. Inorganic Chemicals Industry, 2024, 56(4): 1-7. |
| [3] | FENG Xia, YU Xuefeng, YAO Zhihao, LUO Jun, REN Siying, ZHAO Zhixing, YU Xudong. Study on phase equilibria of aqueous ternary system of Na+(Mg2+),Ca2+∥Cl--H2O at 278.2 K [J]. Inorganic Chemicals Industry, 2024, 56(1): 47-52. |
| [4] | JIN Fang,LI Hongpu,CHANG Donghai. Experiment on natural evaporation of brine in Nanyishan anticline structural area of Qaidam Basin [J]. Inorganic Chemicals Industry, 2021, 53(11): 86-90. |
| [5] | Shi Xingxing,Hu Kaibao,Wang Zhanhe,Pan Jingzhong,Cui Jingui,Luo Ruiyin,Du Wei,Tang Na. Study on salt crystallization law of brine frozen mirabilite and frozen mirabilite mother liquor evaporation in Jilantai Salt Lake [J]. Inorganic Chemicals Industry, 2020, 52(6): 54-58. |
| [6] | Wang Jukui,Dong Xingfeng,Zhao Dong,Wang Shiqiang,Guo Yafei,Deng Tianlong. Solid-liquid phase equilibria in quaternary system lithium borate-potassium borate-magnesium borate-water at 308.15 K [J]. Inorganic Chemicals Industry, 2020, 52(5): 27-30. |
| [7] | Peng Lingling,Wei Xuebin,Zhao Weiyong,Liu Ying,Mu Yanzong,Nie Zhen,Wang Yunsheng. Study on natural evaporation of underground potassium-rich brine in Heibei concave [J]. Inorganic Chemicals Industry, 2019, 51(6): 11-16. |
| [8] | Zhao Xiaoli,Fang Li,Zhao Zesen,Cheng Huaigang,Cheng Fangqin. Study on crystal morphology in preparation of aluminum chloride hexahydrate by ammonium aluminum sulfate-hydrogen chloride reaction [J]. Inorganic Chemicals Industry, 2019, 51(5): 28-32. |
| [9] | MA Song-Liang, DONG Guang-Feng, HU Li. Research on impure kainite transformation particle size [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(8): 57-. |
| [10] | WANG Yong-Mei, LI Chun-Li, LIU Liang-Mei. Phase diagram analysis and calculation of salt pan process of Chaerhan salt lake [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(2): 26-. |
| [11] | XIE Shao-Lei, ZHANG Chao, JI 律, CHEN Gao-Qi, JING Yan. Research on freezing crystalization behavior of magnesium sulfate subtypes brine at low temperature [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(2): 35-. |
| [12] | LIU Huan, ZHANG Bing-Zhu, TIAN Ying, ZHANG Xiao-Fei, ZHANG Yang, XU Hong-Bin, ZHANG Yi. Evaporation crystallization of Na2CrO4 from Na2CrO4-NaOH-H2O system [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(5): 19-. |
| [13] | ZHANG Xia, HUANG Xue-Li, HUANG Wen-Ting, REN Xiao-Jing. Study on cooling process of salt-water system with nitrate [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(4): 18-. |
| [14] | LIU Chang-Jian. New clean process for ammonium dichromate production [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(5): 44-. |
| [15] | FU Zhen-Hai, ZHANG Zhi-Hong, MA Yan-Fang, DONG Sheng-Fa, ZHU Jian-Rong, YAN Zhi-Shuo. Freeze sulfate-type brine at low temperature in stages and theoretical study on evaporation and crystallization behavior [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(2): 29-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||