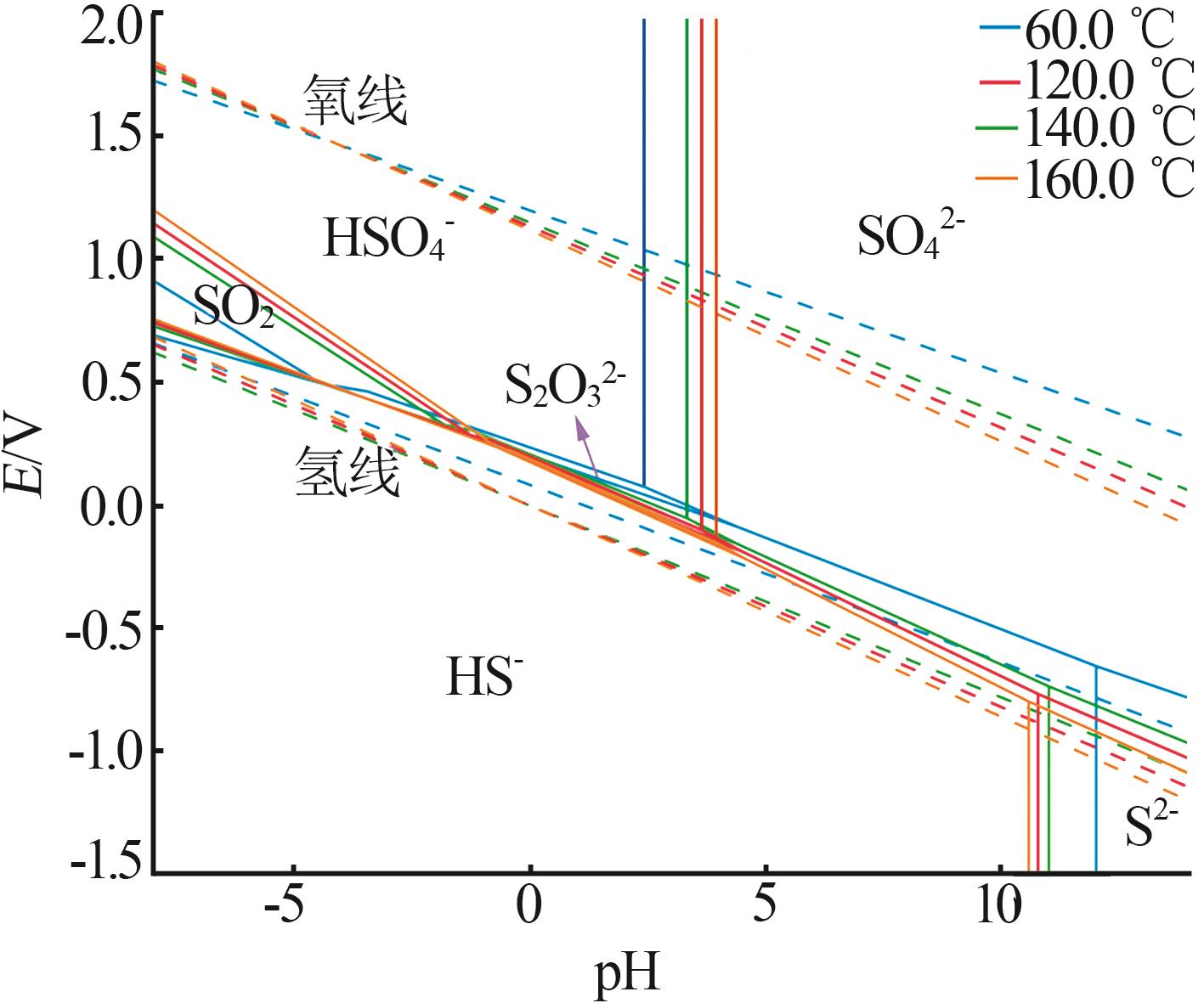
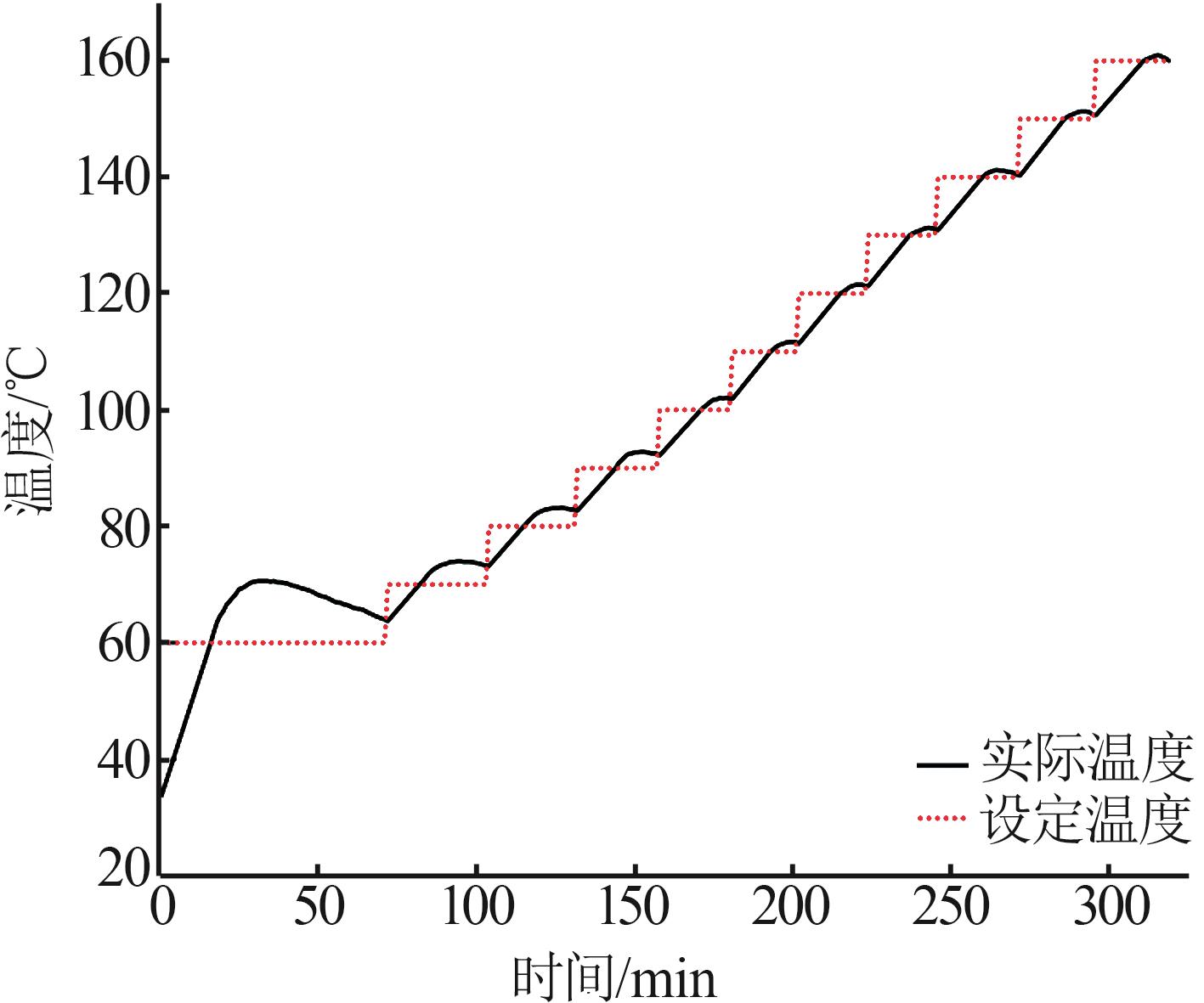
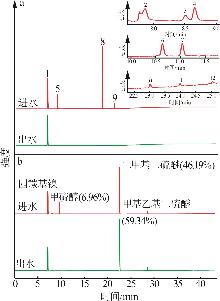
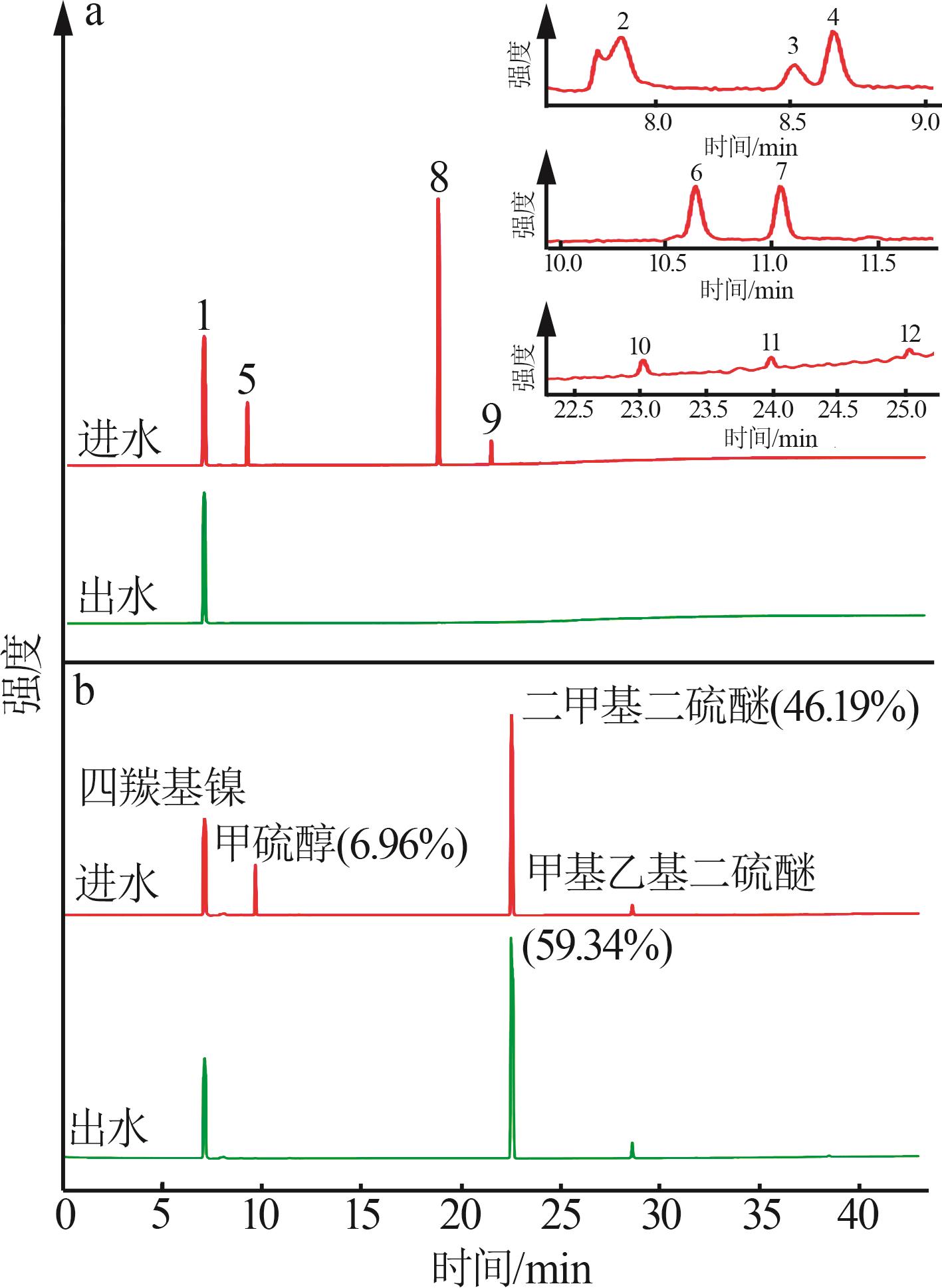
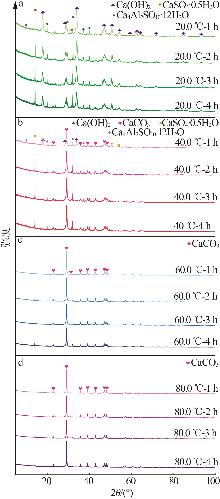
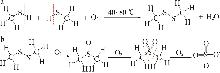Inorganic Chemicals Industry ›› 2025, Vol. 57 ›› Issue (5): 108-115.doi: 10.19964/j.issn.1006-4990.2024-0327
• Environment·Health·Safety • Previous Articles Next Articles
Study on removal of sulphur in alkali residue wastewater liquid by wet oxidation method and its products
SUN Wenyong1( ), WU Yunjiao2,3, WANG Yunxia2, QIN Songyan2, ZHAO Lixin2(
), WU Yunjiao2,3, WANG Yunxia2, QIN Songyan2, ZHAO Lixin2( )
)
- 1. Shandong Dadi Salt Chemical Group Co. ,Ltd. ,Weifang 262700,China
2. Environmental Science and Safety Engineering,Tianjin University of Technology,Tianjin 300384,China
3. Shandong New Scenery Technology Co. ,Ltd. ,Dezhou 253000,China
-
Received:2024-06-11Online:2025-05-10Published:2025-06-05 -
Contact:ZHAO Lixin E-mail:swy369@126.com;collition@sohu.com
CLC Number:
Cite this article
SUN Wenyong, WU Yunjiao, WANG Yunxia, QIN Songyan, ZHAO Lixin. Study on removal of sulphur in alkali residue wastewater liquid by wet oxidation method and its products[J]. Inorganic Chemicals Industry, 2025, 57(5): 108-115.
share this article
| 1 | 陈晓峰,冯瑞军,陈文茜.石油化工碱渣废水的治理措施[J].当代化工,2016,45(4):776-778,781. |
| CHEN Xiaofeng, FENG Ruijun, CHEN Wenqian.Treatment methods of alkaline wastewater from the petrochemical industry[J].Contemporary Chemical Industry,2016,45(4):776-778,781. | |
| 2 | 王滔.废碱液氧化处理技术及进展浅析[J].山东化工,2021,50(14):75-76. |
| WANG Tao.Analysis of oxidation treatment technology and progress of waste alkali liquid[J].Shandong Chemical Industry,2021,50(14):75-76. | |
| 3 | 周翔.焦化液化气脱硫醇系统节能减排及效果分析[J].石化技术,2024,31(4):274-276. |
| ZHOU Xiang.Energy saving,emission reduction and effect analysis of desthiol system of coking liquefied gas[J].Petrochemical Industry Technology,2024,31(4):274-276. | |
| 4 | 蒋晓军.污水处理场WAR系统处理炼油碱渣探讨[J].广东化工,2023,50(2):109-111. |
| JIANG Xiaojun.Discussion on treatment of alkali residue from oil refining with WAR system in sewage treatment plant[J].Guangdong Chemical Industry,2023,50(2):109-111. | |
| 5 | 吴蓬,张涛,耿玉倩,等.碱渣的理化性质及应用研究进展[J].中国粉体技术,2022,28(1):35-42. |
| WU Peng, ZHANG Tao, GENG Yuqian,et al.Physicochemical properties and application research progress of soda residue[J].China Powder Science and Technology,2022,28(1):35-42. | |
| 6 | 刘继艳,牟洪祥,隋立华,等.废胺液处理技术研究进展[J].化工环保,2024,44(6):769-779. |
| LIU Jiyan, MU Hongxiang, SUI Lihua,et al.Research progress on treatment technologies of waste amine solution[J].Environmental Protection of Chemical Industry,2024,44(6):769-779. | |
| 7 | 杜松,张超,吴唯民,等.深井灌注技术用于处理煤矿高盐废水的展望[J].中国给水排水,2020,36(16):40-48. |
| DU Song, ZHANG Chao, WU Weimin,et al.Prospect of deep well injection for treatment of coal mine drainage brine wastewater[J].China Water & Wastewater,2020,36(16):40-48. | |
| 8 | 李玉林,杜善明,吴国祥,等.浅析焚烧技术在煤制烯烃废碱液处理中的应用[J].神华科技,2017,15(8):93-96. |
| LI Yulin, DU Shanming, WU Guoxiang,et al.Analysis of inceneration technology′s application in coal⁃to⁃olefin waste alkali treatment[J].Shenhua Science and Technology,2017,15(8):93-96. | |
| 9 | 杨海燕,朱万学,巴爱叶.石油化工企业碱渣废水治理技术探析[J].全面腐蚀控制,2017,31(12):15-18,55. |
| YANG Haiyan, ZHU Wanxue, BA Aiye.Discussion on treatment technology of alkali residue wastewater in petrochemical enterprises[J].Total Corrosion Control,2017,31(12):15-18,55. | |
| 10 | 李俊,龚德伟,刘晓晶,等.生物强化技术处理废碱液的应用研究[J].化学工程,2021,49(11):11-14. |
| LI Jun, GONG Dewei, LIU Xiaojing,et al.Application research on the treatment of waste alkali by bio⁃enhancement technology[J].Chemical Engineering (China),2021,49(11):11-14. | |
| 11 | 江淦福,褚建益,张文成,等.生物增效强化技术在碱渣废水处理中的应用[J].中国环保产业,2023(12):46-50. |
| JIANG Ganfu, CHU Jianyi, ZHANG Wencheng,et al.Application of bio⁃enhancement technology in treatment of refining alkali slag wastewater[J].China Environmental Protection Industry,2023(12):46-50. | |
| 12 | 付柯,赵强,冯想红,等.高炉煤气精脱硫工艺技术研究进展[J].工业炉,2023,45(6):18-22. |
| FU Ke, ZHAO Qiang, FENG Xianghong,et al.Research progress on fine desulfurization technology of blast furnace gas[J].Industrial Furnace,2023,45(6):18-22. | |
| 13 | 杨爽,王雪峰,范雪健,等.高级氧化技术的研究现状及发展展望[J].工业催化,2024,32(2):26-33. |
| YANG Shuang, WANG Xuefeng, FAN Xuejian,et al.Research status and development prospect of advanced oxidation technology[J].Industrial Catalysis,2024,32(2):26-33. | |
| 14 | 秦树林,王忠泉,郑威城,等.湿式氧化技术在煤化工污泥处理中的应用探析[J].广东化工,2023,50(22):91-92,132. |
| QIN Shulin, WANG Zhongquan, ZHENG Weicheng,et al.Application of wet oxidation technology in coal chemical sludge treatment[J].Guangdong Chemical Industry,2023,50(22):91-92, 132. | |
| 15 | 姚浩,杨四齐,白希为,等.不同温度下Se-H2O系电位-pH图的研究[J].有色金属工程,2024,14(2):49-56. |
| YAO Hao, YANG Siqi, BAI Xiwei,et al.The research of potential-pH diagram of Se-H2O system at different temperatures[J].Nonferrous Metals Engineering,2024,14(2):49-56. | |
| 16 | GAUDIN T, AUBRY J M.Prediction of pourbaix diagrams of quinones for redox flow battery by COSMO-RS[J].Journal of Energy Storage,2022,49:104152. |
| 17 | ZHANG Lehua, DE SCHRYVER P, DE GUSSEME B,et al.Chemical and biological technologies for hydrogen sulfide emission control in sewer systems:A review[J].Water Research,2008,42(1/2):1-12. |
| 18 | LI Yiming, HE Yanying, GUO Haixiao,et al.Sulfur⁃containing substances in sewers:Transformation,transportation,and remediation[J].Journal of Hazardous Materials,2024,467:133618. |
| 19 | ZHOU Changhui, LI Jinhua, ZHANG Yan,et al.A novel SO3·- mediated photoelectrocatalytic system based on MoS2/Fe2O3 and CuNW@CF for the efficient treatment of sulfurous and nitrogenous oxides[J].Applied Catalysis B:Environmental,2023,330:122579. |
| 20 | 李军宏.氧在水中传质过程的探讨[J].广东化工,2014,41(3):69-70,78. |
| LI Junhong.On mass transfer of oxygen in water[J].Guangdong Chemical Industry,2014,41(3):69-70,78. | |
| 21 | 王丹.高含硫废水受控氧化及单质硫的形成特性研究[D].成都:西南石油大学,2015.WANG Dan.Study on controlled oxidation and formation characteristics of elemental sulfur in wastewater with high sulfur conte⁃nt[D].Chengdu:Southwest Petroleum University,2015. |
| 22 | WANG Yaru, LIU Zhiqiang, LV Yeqing,et al.Oxidation of sulfide with the CuO catalyst assisted oxygen microbubbles in alkaline wastewater:Efficiency,sulfur conversion,and mechanisms[J].Chemical Engineering Journal Advances,2022,12:100408. |
| 23 | 代明星.甲硫醇钠制DMDS催化剂的制备及其性能研究[D].重庆:重庆理工大学,2017. |
| DAI Mingxing.Studies on the synthesis and properties of catalyst for sodium thiomethoxide preparing DMDS[D].Chongqing:Ch⁃ongqing University of Technology,2017. | |
| 24 | 王炯,宋亚明.甲硫醇氧化法制二甲基二硫(DMDS)工艺[J].化工管理,2016(18):102. |
| WANG Jiong, SONG Yaming.Methyl mercaptan oxidation dimethyl disulfide(DMDS) process[J].Chemical Enterprise Management,2016(18):102. | |
| 25 | CARLSSON A, RAJANI J.New options for mercaptans remov⁃al[J].Hydrocarbon Engineering,1999,10:23-26. |
| 26 | 柳敬娟.液化气硫醇醚化及二烯烃选择性加氢双功能催化剂的研究[D].北京:中国石油大学(北京),2022. |
| LIU Jingjuan.Study on bifunctional catalyst for mercaptan etherification and diolefin removal in LPG[D].Beijing:China University of Petroleum (Beijing),2022. | |
| 27 | 程新.焦化轻苯中硫氯的影响因素与控制理论研究[D].武汉:武汉科技大学,2023. |
| CHENG Xin.Research on influencing factors and control theory of sulfur and chlorine in coking light benzol[D].Wuhan:Wuhan University of Science and Technology,2023. | |
| 28 | 刘成.甲硫醇氧化法制取二甲基二硫工艺[J].中国化工贸易,2021(6):89-90. |
| LIU Cheng.Preparation of dimethyl disulfide by oxidation of methyl mercaptan[J].China Chemical Trade,2021(6):89-90. | |
| 29 | 王春燕.甲硫醇合成二甲基二硫醚反应动力学研究与反应器设计[D].重庆:重庆大学,2014. |
| WANG Chunyan.The research of process and design of dimethyl disulfide by methyl mercaptan and sulfur[D].Chongqing:Ch⁃ ongqing University,2014. | |
| 30 | 滕弋非,鲁萌,贾喆,等.中高硫煤NaOH-H2O2电解脱硫机理研究[J].安全与环境学报,2024,24(11):4424-4433. |
| TENG Yifei, LU Meng, JIA Zhe,et al.Investigating the mechanism of NaOH-H2O2 electrolytic desulfurization of medium⁃high sulfur coal[J].Journal of Safety and Environment,2024,24(11):4424-4433. | |
| 31 | 李素,余志群.硫醚氧化为亚砜和砜的研究进展[J].浙江化工,2024,55(3):12-17. |
| LI Su, YU Zhiqun.Research progress on the oxidation of thioether to sulfoxide and sulfone[J].Zhejiang Chemical Industry,2024,55(3):12-17. | |
| 32 | 穆廷桢,杨茂华,邢建民.有机硫对生物脱硫过程的抑制机理及其研究进展[J].生物工程学报,2021,37(2):461-472. |
| MU Tingzhen, YANG Maohua, XING Jianmin.Inhibition of biological desulfurization by organosulfur:A review[J].Chinese Journal of Biotechnology,2021,37(2):461-472. | |
| 33 | ZHANG Tong, ZHANG Jintao, WANG Zhi,et al.Review of electrochemical oxidation desulfurization for fuels and minerals[J].Fuel,2021,305:121562. |
| 34 | HOUDA S, LANCELOT C, BLANCHARD P,et al.Oxidative desulfurization of heavy oils with high sulfur content:A review[J].Catalysts,2018,8(9):344. |
| 35 | IZADI R, ASSARIAN D, ALTAEE A,et al.Investigation of methods for fuel desulfurization wastewater treatment[J].Chemical Engineering Research and Design,2023,190:198-219. |
| 36 | 李吉辉.乙烯废碱液脱硫再生技术研究[D].大庆:东北石油大学,2015. |
| LI Jihui.Research on technologies of desulfurization regeneration of spent caustic of ethylene cracking[D].Daqing:Northeast Petroleum University,2015. | |
| 37 | 韩东,张强,张敏.含碱硅酸钠溶液的回收和利用工艺研究[J].无机盐工业,2022,54(2):106-110. |
| HAN Dong, ZHANG Qiang, ZHANG Min.Study on recovery and utilization process of alkali containing sodium silicate solution[J].Inorganic Chemicals Industry,2022,54(2):106-110. | |
| 38 | 刘小波.乙烯废碱液苛化—结晶组合工艺技术研究[D].大庆:大庆石油学院,2006. |
| LIU Xiaobo.A study on causticization—crystallization combined technology of spent caustic from ethylene plant[D].Daqing:Daqing Petroleum Institute,2006. |
| [1] | YANG Yue, ZHU Ganyu, ZHANG Jianbo, MENG Ziheng, LIU Xinhui, YANG Jing, YAN Kun, PENG Zonggui, WANG Qiujian, LI Huiquan. Study on preparation of desulfurizer and byproduct gypsum from calcium carbide slag by cyclone separation [J]. Inorganic Chemicals Industry, 2023, 55(5): 78-84. |
| [2] | YIN Huibin,LI Jun,ZHENG Zhuochao. Research on deep purification process of glauberite gypsum [J]. Inorganic Chemicals Industry, 2022, 54(11): 104-111. |
| [3] | ZENG Ying,YAN Bo,RUAN Yunchun,ZHANG Tao,TIAN Rendao,HU Hong,NIE Dengpan. Study on optimization of technological conditions for preparing α high-strength gypsum from phosphogypsum by crystal conversion [J]. Inorganic Chemicals Industry, 2022, 54(11): 112-117. |
| [4] | ZENG Yanjie,XU Tongfei,HUANG Ziliang,CHEN Zhipeng,LIU Xudong,YI Meigui. Study on removal of calcium impurities from magnesium waste residue by roasting-hydration complexing [J]. Inorganic Chemicals Industry, 2022, 54(11): 118-123. |
| [5] | PAN Zude,LIU Qi,CAO Yang,CHEN Qianlin,YANG Min,XIE Yan. Study on preparation and properties of phosphogypsum based mine filling materials [J]. Inorganic Chemicals Industry, 2022, 54(11): 90-95. |
| [6] | Sun Shuangshuang,Zhong Jianchu,Wang Hongzhi. Study on desiliconization process of copper smelting slag [J]. Inorganic Chemicals Industry, 2021, 53(9): 83-87. |
| [7] | Hang Meiyan,Peng Yajuan,Liu Xinxin,Zhang Haiyan,Tao Xu. Quantitative study on influence of ferrochrome slag modification based on mortar strength decoupling method [J]. Inorganic Chemicals Industry, 2021, 53(1): 72-76. |
| [8] | Yan Xin,Wu Jianyi,Lu Yunfeng,Yan Ziteng. Study on new technology of comprehensive utilization of alkali residue in ammonia alkali plant [J]. Inorganic Chemicals Industry, 2021, 53(1): 68-71. |
| [9] | Chen Gang,Wen Yanshen,Peng Juan,Chen Changming,Gong Chuangzhou,Zhao Jie. Current situation and development direction of waste electronic chemical treatment and disposal standard in China [J]. Inorganic Chemicals Industry, 2020, 52(7): 18-21. |
| [10] | Zhang Bingbing,Ren Jianpo,Shen Zhihong,Jiang Zhiqiang. Experimental study on tail gas absorption in synthesis of 2,3-pyridinedicarboxylic acid [J]. Inorganic Chemicals Industry, 2020, 52(7): 74-76. |
| [11] | Wu Mian,Zou Lanmei,Yu Shaoming. Study on separating iron and rare earth by ammonium jarosite method from acid leach liquor of NdFeB secondary waste [J]. Inorganic Chemicals Industry, 2020, 52(6): 68-71. |
| [12] | Liu Junxia,Yang Yanmeng,Wang Shuaiqi,Liu Pan,Zhang Maoliang,Hai Ran. Effect of chemical activator on mechanical property of red mud geopolymer mortar [J]. Inorganic Chemicals Industry, 2020, 52(6): 72-75. |
| [13] | Peng Wenbo,Yun Jianjun,Ding Bangchao,Bai Zuguo,Xiao Weiyi,Wang Xiaohu. Study on membrane integration technology in treatment of wastewater from chlorinated titanium dioxide [J]. Inorganic Chemicals Industry, 2020, 52(6): 76-79. |
| [14] | Huang Xianghao,Ke Changmei,Yang Jintang,Chen Mei,Ke Haibo,Yu Jinzhao. Study on gold leaching from discarded computer circuit boards by thiourea process [J]. Inorganic Chemicals Industry, 2019, 51(6): 62-64. |
| [15] | LI Jian-Wei, YANG Jiu-Jun, WANG Xiao, ZHANG Mao-Liang, HAN Yu-Fang, LUO Zhong-Tao. Research on resource characteristics of red mud from sintering process [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(3): 42-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||