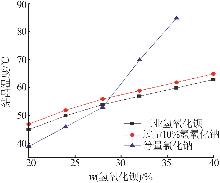
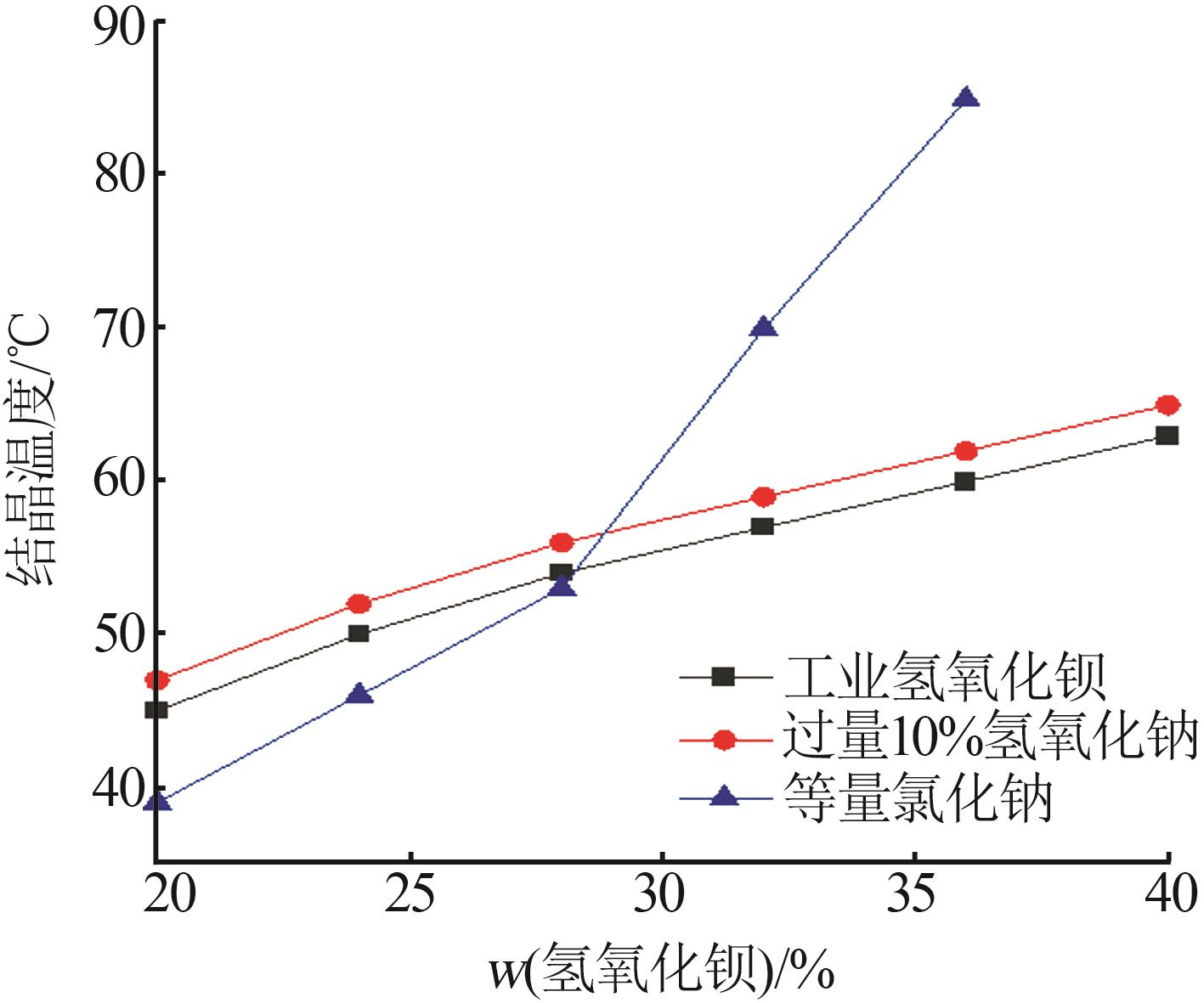
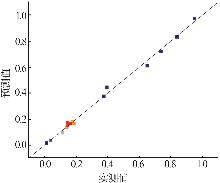
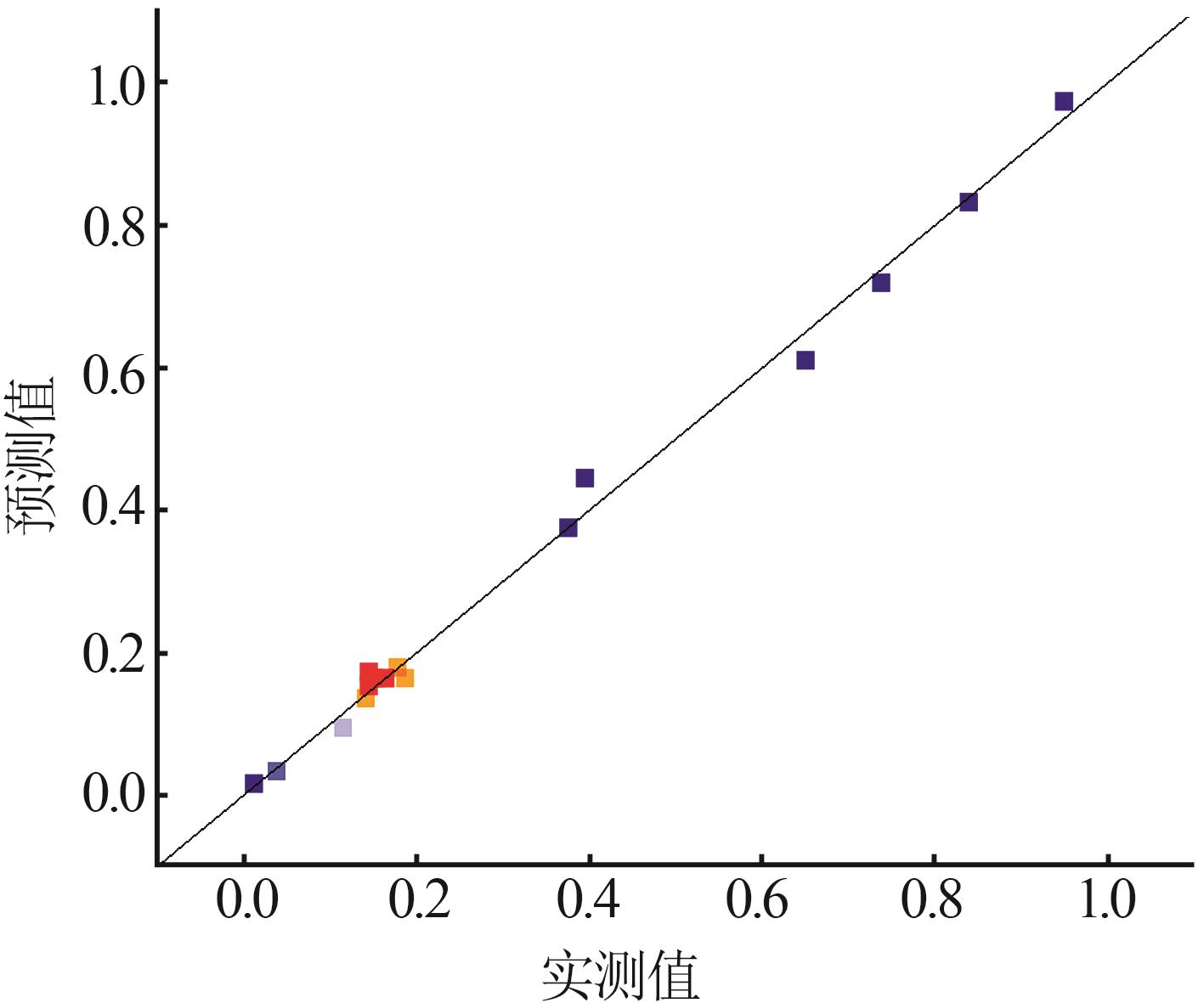
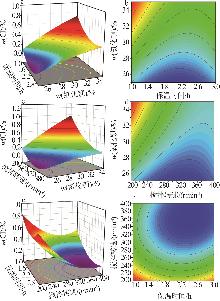
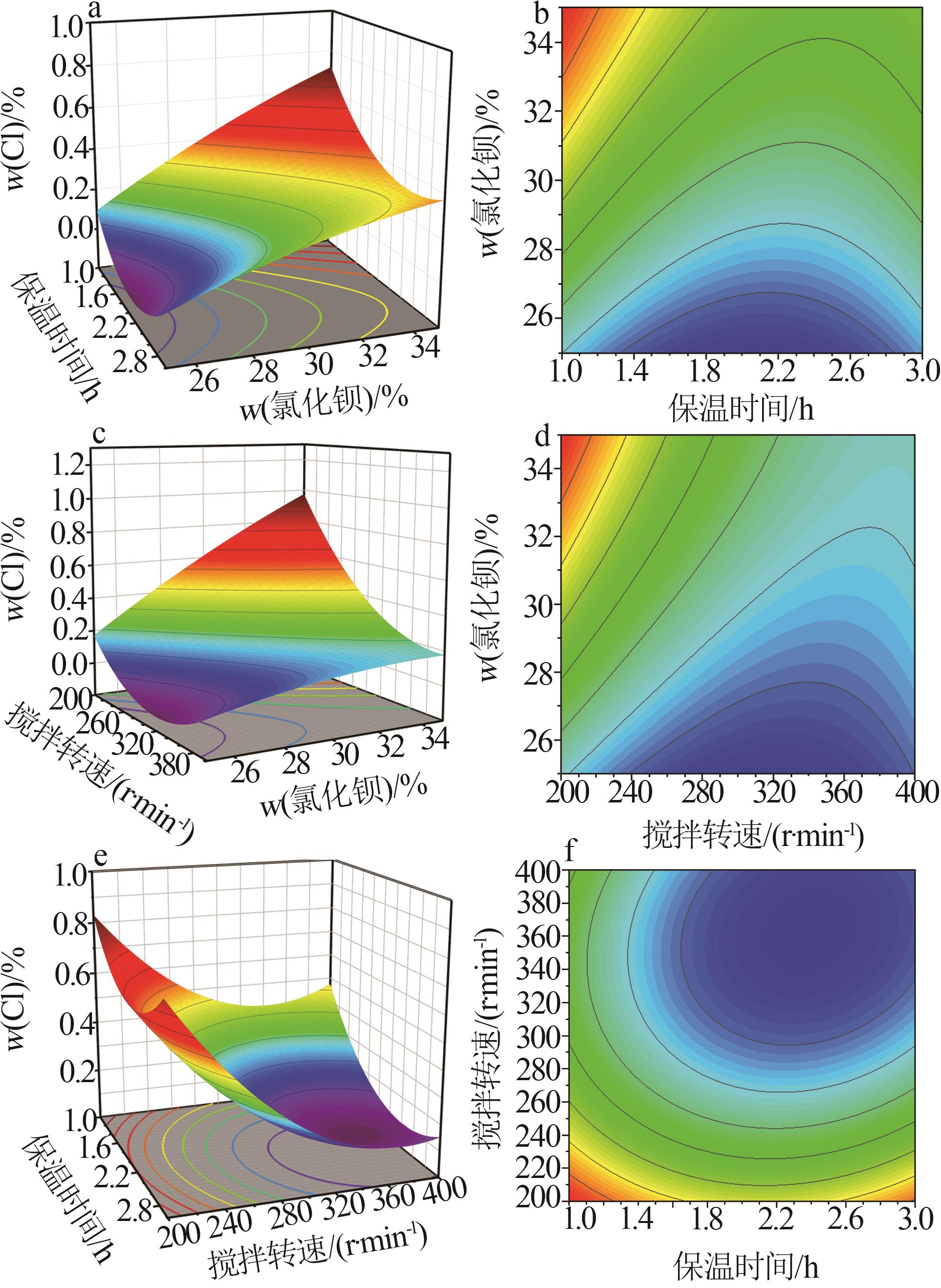
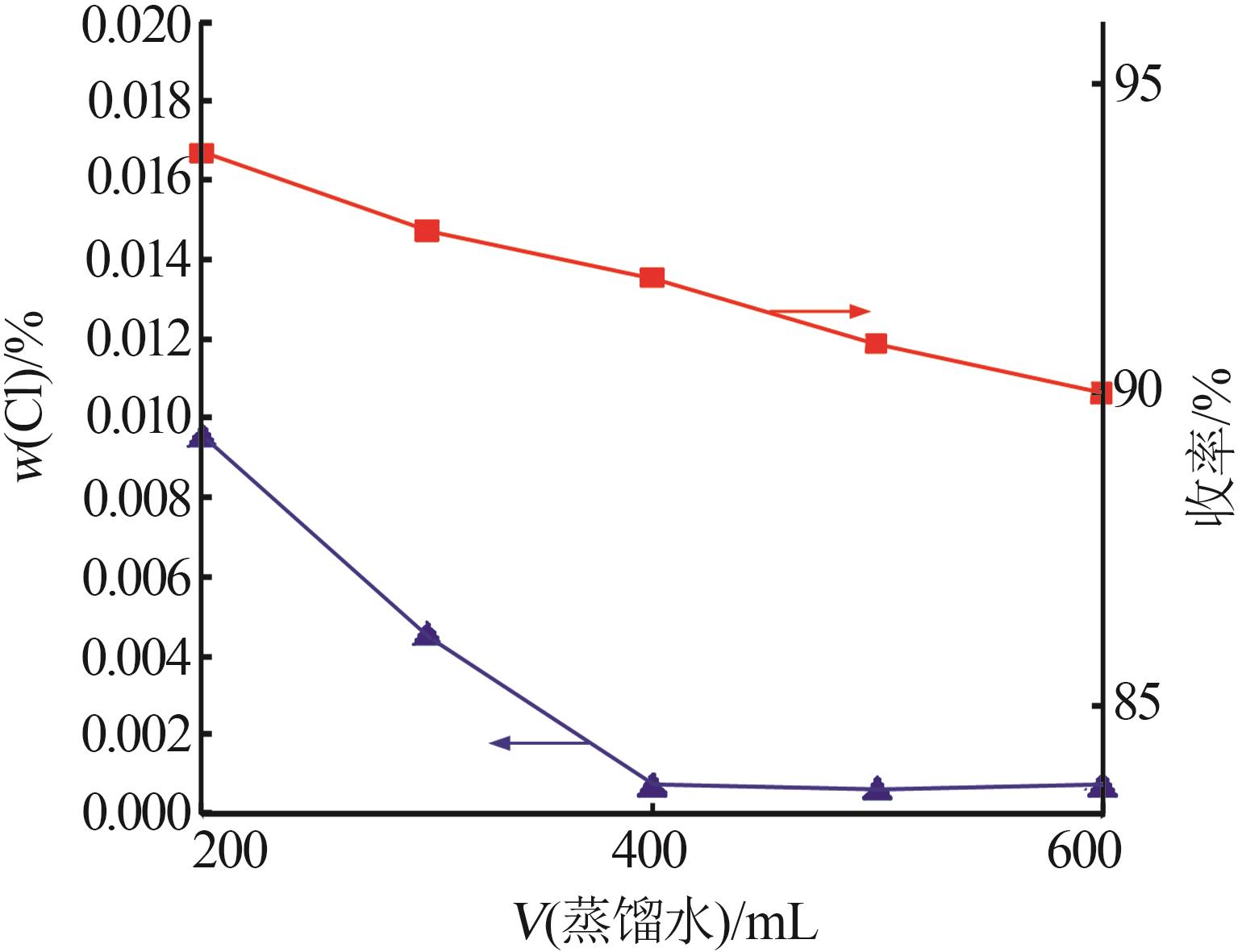
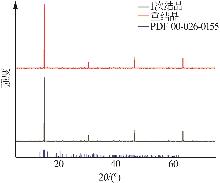
| [1] |
韩茵,朱归胜,徐华蕊,等.高分散纳米钛酸钡粉体的常压水热制备及性能研究[J].电子元件与材料,2021,40(7):631-636, 642.
|
|
HAN Yin, ZHU Guisheng, XU Huarui,et al.Hydrothermal preparation under atmospheric pressure and properties of highly dispersed nano-barium titanate powders[J].Electronic Components and Materials,2021,40(7):631-636,642.
|
| [2] |
邹清栎,邹建新.钛酸钡基电子陶瓷材料的改性进展[J].无机盐工业,2022,54(6):46-54.
|
|
ZOU Qingli, ZOU Jianxin.Modification progress on barium titanate-based electronic ceramic materials[J].Inorganic Chemicals Industry,2022,54(6):46-54.
|
| [3] |
宁延生.无机盐工艺学[M].北京:化学工业出版社,2013:183-234,373-377.
|
| [4] |
何国新.八水氢氧化钡合成工艺研究[J].四川化工,2013,16(5):7-9.
|
|
HE Guoxin.Study on the synthesis of barium hydroxide octahydra-te[J].Sichuan Chemical Industry,2013,16(5):7-9.
|
| [5] |
许明,李思燃,张媛.钴掺杂钛酸钡的制备及其电催化析氧性能研究[J].吉林师范大学学报(自然科学版),2023,44 (3): 38-42.
|
|
XU Ming, LI Siran, ZHANG Yuan.Preparation of cobalt-doped barium titanate and its application in electrocatalytic oxygen evolution[J].Journal of Jilin Normal University(Natural Science Edition),2023,44 (3): 38-42.
|
| [6] |
吴亚丽,吴怡含,张瑞敏,等. 空心钛酸钡的制备及其形成机理 [J].无机盐工业, 2021,53(2): 51-54.
|
|
WU Yali, WU Yihan, ZHANG Ruimin,et al. Preparation and formation mechanism of hollow barium titanate[J].Inorganic Chemicals Industry,2021,53(2): 51-54.
|
| [7] |
WANG Qian, WANG Jiangtao, CHEN Yunyu,et al.Experimental investigation of barium hydroxide octahydrate as latent heat storage materials[J].Solar Energy,2019,177:99-107.
|
| [8] |
ZHAO Sitong, YAN Suying, MING Tingzhen,et al.Experimental study of the preparation and modification of Ba(OH)2·8H2O high-performance composite phase change materials[J].Journal of Ther-
|
|
mal Analysis and Calorimetry,2022,147(23):13239-13252.
|
| [9] |
程利山,苏雪桐,尚苏滢.工业氯化钡生产工艺研究进展及趋势[J].山东化工,2022,51(15):83-85,89.
|
|
CHENG Lishan, SU Xuetong, SHANG Suying.Research progress and development trend on production process of industrial barium chloride[J].Shandong Chemical Industry,2022,51(15):83-85,89.
|
| [10] |
叶铁林.化工结晶过程原理及应用[M].3版.北京:北京工业大学出版社,2020.
|
| [11] |
章聪华,颜文斌,肖佳俊,等.响应面法优化酒石酸还原浸出电解锰阳极渣工艺[J].无机盐工业,2023,55(9):106-113.
|
|
ZHANG Conghua, YAN Wenbin, XIAO Jiajun,et al.Reductive leaching technology of manganese anode slag using tartaric acid as reducing agent optimized by RSM[J].Inorganic Chemicals Industry,2023,55(9):106-113.
|
| [12] |
张志强,崔康平,陈星,等.响应曲面法优化碳热还原重铬酸钠制备超细氧化铬[J].无机盐工业,2023,55(10):136-144.
|
|
ZHANG Zhiqiang, CUI Kangping, CHEN Xing,et al.Optimization of preparation of ultrafine chromium oxide by carbothermal reduction of sodium dichromate by response surface methodolo-gy[J].Inorganic Chemicals Industry,2023,55(10):136-144.
|
| [13] |
SONG Keliang, JIANG Zhuoni, HE Fangfang,et al.Supercooling degree decrease of barium hydroxide octahydrate employing 3D porous silver foam as supporting material[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2024,684:133138.
|
| [14] |
DING Houyuan, LIN Liangliang, GU Dan,et al.Synthesis and surface modification of submicron BaTiO3 powders via a facile surfactant-assisted method[J].Ceramics International,2020,46(14):22040-22048.
|
| [15] |
ZHANG Yudi, ZHANG Xuelai, JI Jun.Experimental study on powdered expansion of barium hydroxide octahydrate crystal[J].Journal of Molecular Liquids,2019,296:111907.
|
| [16] |
PANG Xiaoxiao, WANG Tingting, LIU Bin,et al.Effect of solvents on the morphology and structure of barium titanate synthesized by a one-step hydrothermal method[J].International Journal of Minerals,Metallurgy and Materials,2023,30(7):1407-1416.
|
 ), JIANG Wenwei1(
), JIANG Wenwei1( ), YE Xianyu2, PENG Xiaoshuang1
), YE Xianyu2, PENG Xiaoshuang1