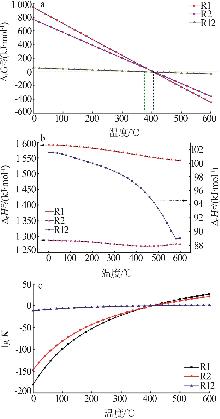
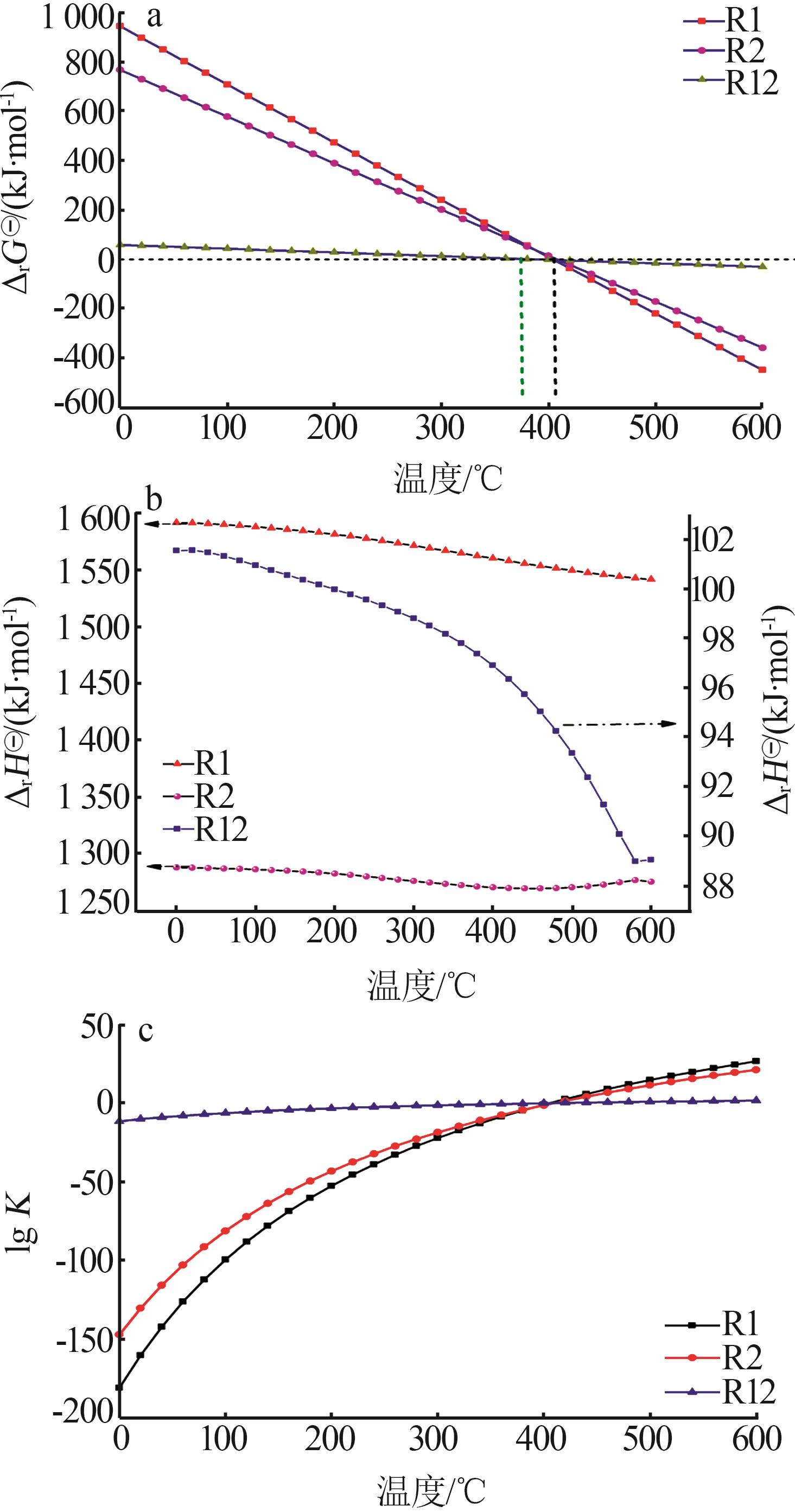
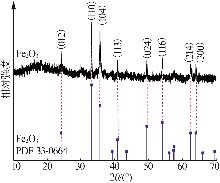
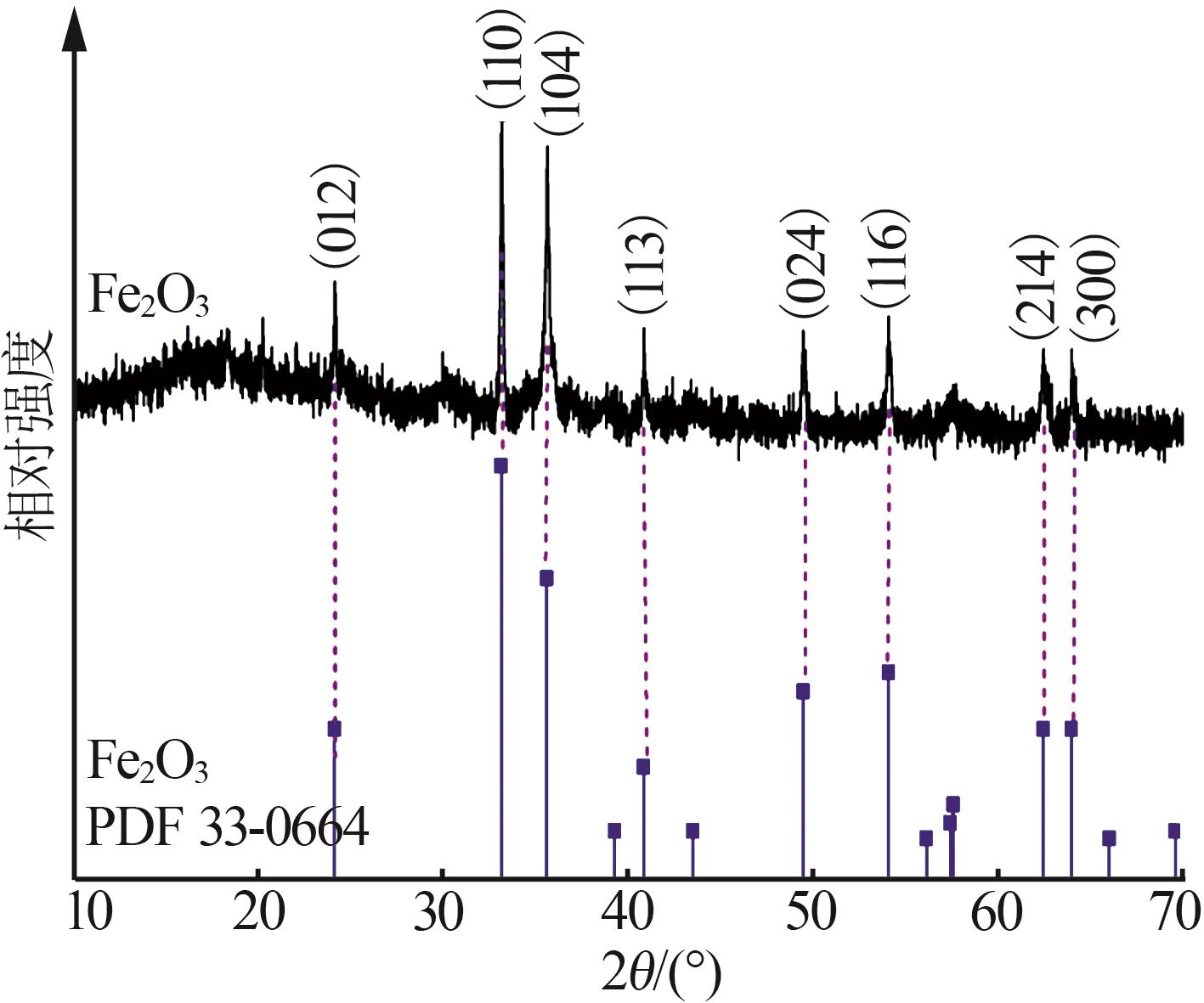
| [1] |
李丹萍.纳米氧化铁的可控制备、结构调控及气敏性能研究[D].杭州:中国计量大学,2017.
|
|
LI Danping.The controllable synthesis,tunable structure and gas-sensing properties of iron oxides[D].Hangzhou:China Jiliang University,2017.
|
| [2] |
杜庆波.氧化铁纳米材料的制备、表征及磁性研究[J].硅酸盐通报,2016,35(9):2922-2924,2929.
|
|
DU Qingbo.Preparation,characterization and magnetic properties of iron oxide nanomaterials[J].Bulletin of the Chinese Ceramic Society,2016,35(9):2922-2924,2929.
|
| [3] |
YANG Daming, SUN Guiru, WANG Xinru,et al.α-Fe2O3 nanospindles as an efficient catalyst for optical and magnetic fields co-assisted Li-O2 cells[J].Chemical Engineering Journal,2023,474:145712.
|
| [4] |
LU P A, MANIKANDAN M R, YANG P F,et al.Synthesis,analysis and characterization of alpha-Fe2O3 nanoparticles and their applications in supercapacitors[J].Journal of Materials Science:Materials in Electronics,2023,34(9):826.
|
| [5] |
PARK C, JUNG J, LEE C W,et al.Synthesis of mesoporous α-Fe2O3 nanoparticles by non-ionic soft template and their applications to heavy oil upgrading[J].Scientific Reports,2016,6:39136.
|
| [6] |
ZHANG Yong, XIAO Yifan, XU Guangsong,et al.Preparation of Fe2O3 porous microspheres modified pumice and its adsorption performance on phosphate removal[J].Journal of Environmental Chemical Engineering,2023,11(3):109995.
|
| [7] |
NAJAFI KANI E, RAFIEAN A H, ALISHAH A,et al.The effects of nano-Fe2O3 on the mechanical,physical and microstructure of cementitious composites[J].Construction and Building Materials,2021,266:121137.
|
| [8] |
HUANG Wei, LU Xiaoyu, JIA Dongsheng,et al.Characterization of structural,optical and photocatalytic properties of yttrium modified hematite(α-Fe2O3) nanocatalyst[J].Ceramics International,2023,49(15):25602-25611.
|
| [9] |
SAJJAD A, HUSSAIN S, JAFFARI G H,et al.Fabrication of hematite(α-Fe2O3) nanoparticles under different spectral lights transforms physio chemical,biological,and nanozymatic properti- es[J].Nano Trends,2023,2:100010.
|
| [10] |
吴文军,韩召,张福元,等.高纯纳米氧化铁的制备[J].中国粉体技术,2024,30(1):56-65.
|
|
WU Wenjun, HAN Zhao, ZHANG Fuyuan,et al.Preparation of high-purity nano iron oxide[J].China Powder Science and Technology,2024,30(1):56-65.
|
| [11] |
胡军英,江民华,刘嘉欣,等.氧化铁纳米材料的制备及其光催化性能研究进展[J].江西化工,2023,39(3):1-6,12.
|
|
HU Junying, JIANG Minhua, LIU Jiaxin,et al.Research progress on preparation and photocatalysis performance of iron oxide nanomaterials[J].Jiangxi Chemical Industry,2023,39(3):1-6,12.
|
| [12] |
陈瑶姬,宋可鑫,程微,等.乙酸铁热分解制备磁性纳米氧化铁及对砷的吸附性能[J].环境化学,2024,43(12):4303-4311.
|
|
CHEN Yaoji, SONG Kexin, CHENG Wei,et al.Preparation of magnetic nano iron oxide by thermal decomposition of ferric acetate and its adsorption performance for arsenic[J].Environmental Chemistry,2024,43(12):4303-4311.
|
| [13] |
杨信成,薛永强.纳米氧化铁的制备及其相变研究[J].辽宁化工,2020,49(10):1253-1255,1265.
|
|
YANG Xincheng, XUE Yongqiang.Study on preparation and phase transition of nanometer iron oxide[J].Liaoning Chemical Industry,2020,49(10):1253-1255,1265.
|
| [14] |
SARANGI P P, NAIK B, GHOSH N N.Low temperature synthesis of single-phase α-Fe2O3 nano-powders by using simple but novel chemical methods[J].Powder Technology,2009,192(3):245-249.
|
| [15] |
杨幸川,位根磊,徐丽,等.己二酸二甲酯加氢反应热力学分析及动力学研究[J].化工学报,2021,72(5):2465-2473.
|
|
YANG Xingchuan, WEI Genlei, XU Li,et al.Thermodynamic analysis and kinetic study on hydrogenation of dimethyl adipate [J].CIESC Journal,2021,72(5):2465-2473.
|
| [16] |
孙玉洁,景旋,胡利如,等.对苯二甲酸酯化合成对苯二甲酸二甲酯的反应热力学计算及分析[J].合成技术及应用,2023,38(4):51-54,58.
|
|
SUN Yujie, JING Xuan, HU Liru,et al.Dimethyl terephthalate synthesis from methyl terephthalate:Thermodynamic computation and analysis[J].Synthetic Technology & Application,2023,38(4):51-54,58.
|
| [17] |
HUANG Penghui, JIANG Bing, ZHANG Zhiye,et al.Recycling sulfur and iron resources in the waste ferrous sulfate[J].Journal of Thermal Analysis and Calorimetry,2015,119(3):2229-2237.
|
| [18] |
TAO Ye, JIANG Bin, YANG Xiushan,et al.Physicochemical study of the sustainable preparation of nano-Fe2O3 from ferrous sulfate with coke[J].Journal of Cleaner Production,2020,255:120175.
|
| [19] |
张锐,葛泮珠,任亮,等.1-甲基萘加氢饱和反应热力学平衡分析及实验研究[J].石油学报(石油加工),2022,38(6):1281-1290.
|
|
ZHANG Rui, GE Panzhu, REN Liang,et al.Thermodynamic equilibrium analysis and experimental research on hydrogenation saturation reaction of 1-methylnaphthalene[J].Acta Petrolei Sinica (Petroleum Processing Section),2022,38(6):1281-1290.
|
| [20] |
刘宗鹏,胡少剑,张宇宁,等.复合型多元醇酯合成反应的热力学分析及动力学研究[J].化工学报,2023,74(11):4475-4486.
|
|
LIU Zongpeng, HU Shaojian, ZHANG Yuning,et al.Thermodynamic analysis and kinetics study on synthesis reaction of complex polyolester[J].CIESC Journal,2023,74(11):4475-4486.
|
 ), LUO Xin, ZHU Denglei, ZHANG Min
), LUO Xin, ZHU Denglei, ZHANG Min