Inorganic Chemicals Industry ›› 2021, Vol. 53 ›› Issue (4): 56-60.doi: 10.11962/1006-4990.2020-0315
• Research & Development • Previous Articles Next Articles
Study on law of lithium extraction and impurity removal from spodumene leaching solution
Tan Bo,Liu Xianghuan,Liu Xudong,Yi Meigui( )
)
- School of Chemical Engineering, Sichuan University, Chengdu 610065, China
-
Received:2020-10-19Online:2021-04-10Published:2021-04-23 -
Contact:Yi Meigui E-mail:meiguiyi@scu.edu.cn
CLC Number:
Cite this article
Tan Bo,Liu Xianghuan,Liu Xudong,Yi Meigui. Study on law of lithium extraction and impurity removal from spodumene leaching solution[J]. Inorganic Chemicals Industry, 2021, 53(4): 56-60.
share this article
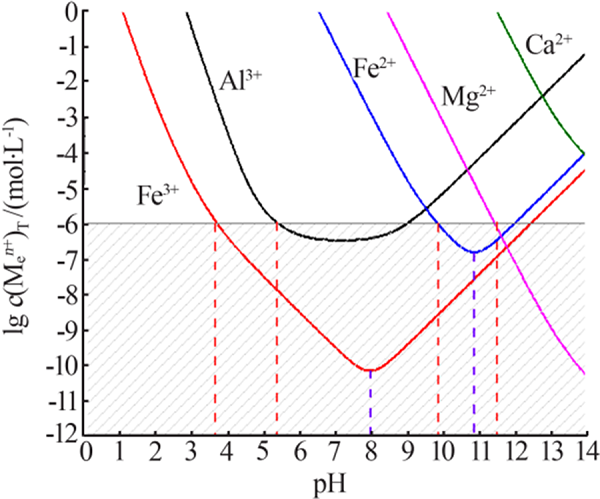
Table 3
Equilibrium constant of Al(OH)3"
| 平衡关系式 | 平衡常数 |
|---|---|
| Al3++OH-=Al(OH)2+ | β1=9.1 |
| Al3++2OH-=Al(OH)2+ | β2=18.7 |
| Al3++3OH-=Al(OH)3(aq) | β3=27.0 |
| Al3++4OH-=Al(OH)4- | β4=33.0 |
| 2Al3++2OH-=Al2(OH)24+ | β2, 2=19.34 |
| 3Al3++4OH-=Al3(OH)45+ | β4, 3=43.2 |
| 13Al3++32OH-=Al13(OH)327+ | β32, 13=345.2 |
| Al(OH)3(s)=Al3++3OH- | lg Ksp=-33.5 |
Table 4
Equilibrium of Fe2+/3+, Mg2+ and Ca2+ in solution"
| 平衡关系式 | 平衡常数 |
|---|---|
| Fe3++OH-=Fe(OH)2+ | β1=11.5 |
| Fe3++2OH-=Fe(OH)2+ | β2=21.3 |
| Fe3++3OH-=Fe(OH)3(aq) | β3=-2.23 |
| Fe3++4OH-= Fe(OH)4- | β4=34.4 |
| 2Fe3++2OH-=Fe2(OH)24+ | β2, 2=20.78 |
| 3Fe3++4OH-=Fe3(OH)45+ | β4, 3=49.9 |
| Fe(OH)3(s)=Fe3++3OH- | lg Ksp= -38.8 |
| Fe2++OH-=Fe(OH)+ | β1=4.68 |
| Fe2++2OH-=Fe(OH)2(aq) | β2=-2.22 |
| Fe2++3OH-=Fe(OH)3- | β3=11.0 |
| Fe2++4OH-=Fe(OH)42- | β4=10.0 |
| Fe(OH)2(s)=Fe2++2OH- | lg Ksp=-14.95 |
| Mg2++OH-=Mg(OH)+ | β1=-6.46 |
| Mg2++2OH-=Mg(OH)2(aq) | β2=-0.066 |
| 2Mg2++OH-=Mg2(OH)3+ | β1, 2=2.38 |
| 4Mg2++4OH-=Mg4(OH)44+ | β4, 4=18.6 |
| Mg(OH)2(s)=Mg2+ +2OH- | lg Ksp=-11.16 |
| Ca2++OH-=Ca(OH) + | β1=1.18 |
| Ca2++2OH-=Ca(OH)2(aq) | β2=0.15 |
| Ca(OH)2(s)=Ca2++2OH- | lg Ksp=-5.41 |
| [1] | 王冬斌, 梁精龙, 邓孝纯 , 等. 锂资源提取与回收及锂制备工艺研究现状[J]. 无机盐工业, 2020,52(6):8-12. |
| [2] | 张永兴, 马亚梦, 张利珍 , 等. 从浓缩盐湖卤水中萃取分离锂的实验研究[J]. 无机盐工业, 2019,51(3):38-40. |
| [3] | 魏昊, 田欢, 张梦龙 , 等. 电池级碳酸锂制备与提纯的研究进展[J]. 现代化工, 2018,38(8):33-37. |
| [4] | 周有池, 文小强, 郭春平 , 等. 铁锂废料制备电池级碳酸锂和磷酸铁工艺研究[J]. 有色金属:冶炼部分, 2019(4):73-77. |
| [5] | 赵光金, 何睦, 唐国鹏 , 等. 废旧磷酸铁锂电池正极材料浸取及回收研究[J]. 电源技术, 2019,43(3):442-444, 452. |
| [6] | 王智娟, 向兰 . 温度对湿法磷酸选择性除杂的影响[J]. 非金属矿, 2020,43(1):22-24. |
| [7] | Ruan Y, Zhang Z, Luo H , et al. Effects of metal ions on the flotation of apatite, dolomite and quartz[J]. Minerals, 2018,8(4):141. |
| [8] | 邹海凤, 程琥, 王雪 , 等. 废旧锂离子电池正极材料中有价金属的浸出回收工艺[J]. 应用化工, 2019,48(6):1366-1369. |
| [1] | An Xiaoying,Huang Wengfang,Wang Zhengli,Peng Yihua,Ye Jiasheng,Li Ming,Lai Xiaoli. Discussion on determination method of zinc constant in waste chemicals [J]. Inorganic Chemicals Industry, 2021, 53(3): 84-86. |
| [2] | Cao Liqiong,Zhang Xiaoxi,Wu Lixiang,Cheng Huaigang,Cheng Fangqin. Experimental investigation of floating phenomenon of carnalliti NaCl in direct flotation process of KCl [J]. Inorganic Chemicals Industry, 2020, 52(7): 26-29. |
| [3] | Xu Xinfang,Zhou Xiaoping,Liu Zhengfeng,Li Lei,Chen Hu,Li Changming. Study on causticization and purification of salt lake lithium ore and recovery of fluorine-containing lithium carbonate [J]. Inorganic Chemicals Industry, 2020, 52(7): 62-65. |
| [4] | Zhang Yan,Zhao Zhenzhong,Ma Zhaohui,Zhao Yancai,Chen Zhiyu,Ma Zhengqiang. Optimization of process for preparation of battery grade lithium carbonate by carbonization [J]. Inorganic Chemicals Industry, 2020, 52(3): 68-71. |
| [5] | Hu Min,Gong Hanzhang,Wu Huadong,Guo Jia,Zhang Linfeng,Zhou Yuxin. Preparation of battery-grade lithium carbonate from lithium-containing industrial waste [J]. Inorganic Chemicals Industry, 2020, 52(3): 80-84. |
| [6] | Han Longnian,Xin Jing,Zhang Ping,Chen Yufei,Fan Wenxuan,Wei Linlin. Analysis of impurity deposition on guard catalyst for diesel hydrocracking device [J]. Inorganic Chemicals Industry, 2020, 52(12): 113-117. |
| [7] | Huang Zhong,Yu Shuangqiang,Gao Kaiyuan,He Jun,Huang He,Zhang Xianfeng,Chen Xizhen. Study on process technology of preparing NH4F and co-producing MgF2 with high impurity fluosilicic acid [J]. Inorganic Chemicals Industry, 2020, 52(10): 110-116. |
| [8] | Liu Gousheng,Wang Linlin,Liu Yuelong. Preparation of lithium carbonate from medium-and low-grade lithium clay by ammonium sulfate process [J]. Inorganic Chemicals Industry, 2020, 52(10): 125-129. |
| [9] | Chen Jie,Chen Xia. Crystallization process optimization for preparation of lithium carbonate [J]. Inorganic Chemicals Industry, 2019, 51(8): 29-32. |
| [10] | Liu Guowang,Zhou Xiaojun,Zhang Shichun,Yang Shangming,Dong Shoulong,Li Binshou. Recycle method of lithium extracted mother solution and phase diagram analysis [J]. Inorganic Chemicals Industry, 2019, 51(6): 38-40. |
| [11] | Fan Tianbo,Jiang Yu,Chen Si,You Gang,Ding Ke,Sun Xiaojun,Liu Luping,Li Li,Liu Yunyi,Hu Kaizhou,Ma Junru,Guo Xiyao. Application of TRIZ theory in preparation of magnesium hydroxide by light burning ammonia [J]. Inorganic Chemicals Industry, 2019, 51(5): 23-27. |
| [12] | Yin Jishuai,Sun Wenliang,Hao Rusi,Wang Xiao. Study on the influence of lithium deposition reaction conditions on purity and impurities of lithium carbonate [J]. Inorganic Chemicals Industry, 2019, 51(3): 29-33. |
| [13] | Hao Ziyang,Yuan Bo,Li Xin,Yi Meigui. Research on removal mechanism of soluble impurities in lithium concentrate [J]. Inorganic Chemicals Industry, 2019, 51(3): 49-52. |
| [14] | Li Xin1,Yuan Bo2,Yi Meigui1. Study on removal of trace sulfur impurities from lithium carbonate by hydrothermal method [J]. Inorganic Chemicals Industry, 2019, 51(11): 28-30. |
| [15] | Hao Jiantang,Wen Fengyuan,Li Xia. Study on extracting lithium from electrolytic aluminum waste residue [J]. Inorganic Chemicals Industry, 2019, 51(10): 69-71. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|