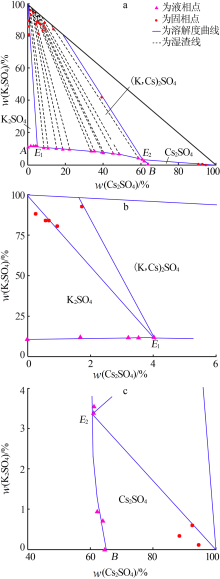
Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (6): 17-20.
• Research & Development • Previous Articles Next Articles
Stable phase equilibrium of the ternary system K +,Cs +//SO4 2--H2O at 298.2 K
Wu Linxin1,Zeng Ying1,2( ),Chen Peijun1,Huang Peng1,Chen Yu1,Sun Jiu1,Yu Xudong1,2
),Chen Peijun1,Huang Peng1,Chen Yu1,Sun Jiu1,Yu Xudong1,2
- 1. College of Materials and Chemistry & Chemical Engineering,Chengdu University of Technology,Chengdu 610059,China
2. Sichuan Collaborative Innovation Center of Panxi Strategic Mineral ResourcesMulti-Purpose Utilization(Chengdu University of Technology)
-
Received:2019-01-14Online:2019-06-10Published:2020-05-12 -
Contact:Zeng Ying E-mail:zengyster@163.com
CLC Number:
Cite this article
Wu Linxin,Zeng Ying,Chen Peijun,Huang Peng,Chen Yu,Sun Jiu,Yu Xudong. Stable phase equilibrium of the ternary system K +,Cs +//SO4 2--H2O at 298.2 K[J]. Inorganic Chemicals Industry, 2019, 51(6): 17-20.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
"
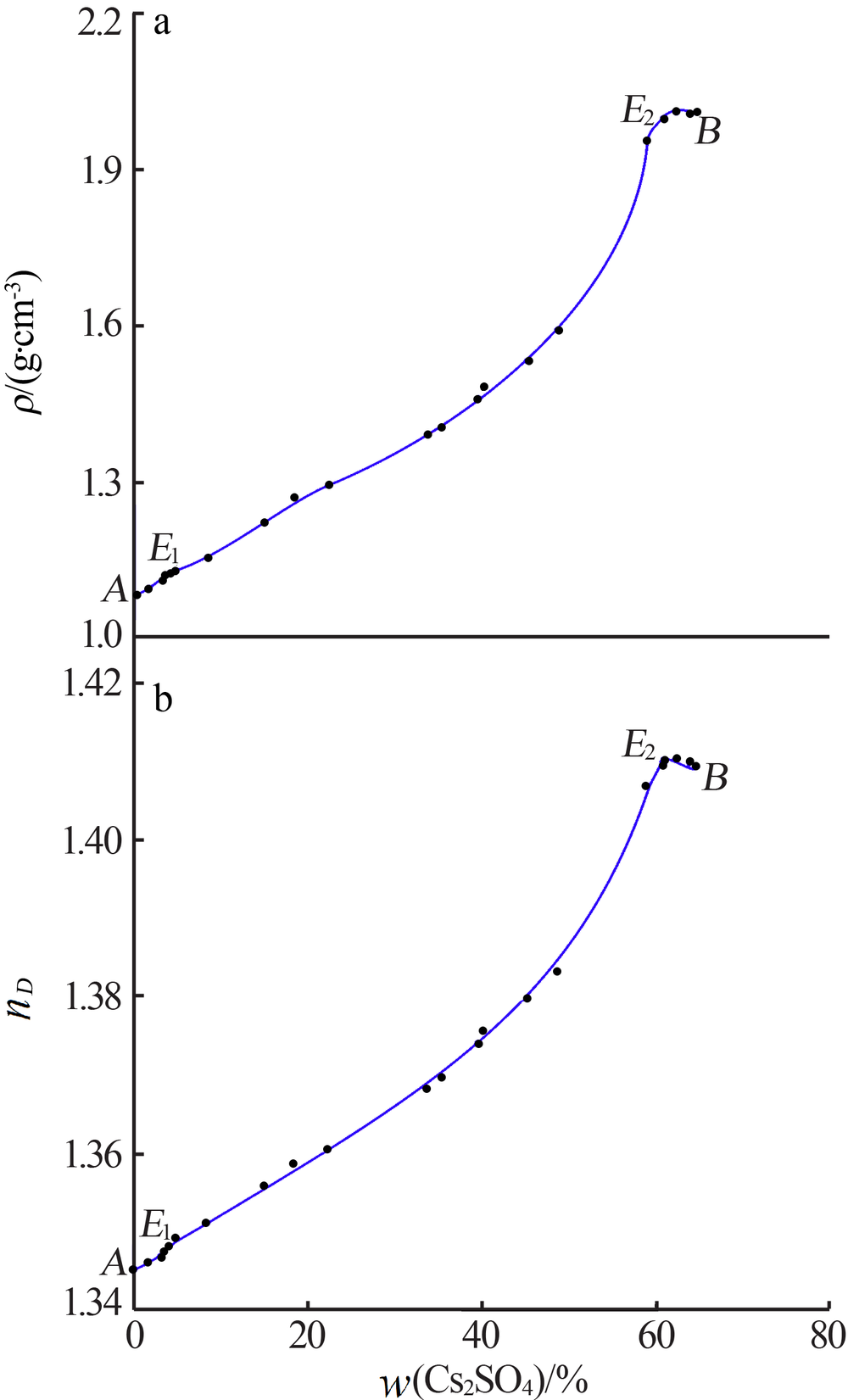
| 编号 | ρ/ (g·cm-3) | nD | 液相组成 w(M)/% | 固相组成 w(M)/% | 平衡 固相 | ||
|---|---|---|---|---|---|---|---|
| K2SO4 | Cs2SO4 | K2SO4 | Cs2SO4 | ||||
| 1,A | 1.081 5 | 1.345 2 | 10.93 | 0.00 | 0.00 | 0.00 | Ar |
| 2 | 1.096 2 | 1.346 1 | 11.93 | 1.68 | 88.32 | 0.27 | Ar |
| 3 | 1.111 9 | 1.346 8 | 11.98 | 3.21 | 84.28 | 0.58 | Ar |
| 4 | 1.119 7 | 1.347 5 | 11.78 | 3.55 | 84.21 | 0.66 | Ar |
| 5,E1 | 1.124 6 | 1.348 1 | 11.69 | 4.02 | 80.50 | 0.94 | Ar+AC |
| 6 | 1.154 9 | 1.351 1 | 10.51 | 8.36 | 92.91 | 1.74 | AC |
| 7 | 1.221 2 | 1.355 9 | 10.10 | 15.01 | 94.24 | 3.10 | AC |
| 8 | 1.271 9 | 1.358 7 | 9.71 | 18.42 | 87.37 | 4.42 | AC |
| 9 | 1.292 5 | 1.360 5 | 9.31 | 22.24 | 88.87 | 4.89 | AC |
| 10 | 1.389 2 | 1.368 2 | 8.72 | 33.69 | 88.44 | 5.70 | AC |
| 11 | 1.405 3 | 1.369 7 | 8.46 | 35.39 | 87.46 | 6.68 | AC |
| 12 | 1.460 2 | 1.374 0 | 7.85 | 39.64 | 86.30 | 8.43 | AC |
| 13 | 1.479 7 | 1.375 7 | 7.53 | 40.17 | 83.31 | 7.93 | AC |
| 14 | 1.531 8 | 1.379 8 | 7.01 | 45.18 | 83.96 | 9.43 | AC |
| 15 | 1.592 4 | 1.383 2 | 6.60 | 48.68 | 87.97 | 9.02 | AC |
| 16 | 1.953 5 | 1.406 9 | 3.95 | 58.92 | 84.12 | 13.34 | AC |
| 17 | 1.998 7 | 1.410 2 | 3.56 | 60.99 | 42.17 | 39.30 | AC |
| 18,E2 | 1.998 5 | 1.409 6 | 3.39 | 60.84 | 0.60 | 92.67 | Cs+AC |
| 19 | 2.008 7 | 1.410 5 | 0.96 | 62.37 | 0.34 | 88.62 | Cs |
| 20 | 2.005 2 | 1.409 9 | 0.71 | 63.96 | 0.11 | 94.57 | Cs |
| 21,B | 2.016 2 | 1.409 5 | 0.00 | 64.63 | 0.00 | 0.00 | Cs |
"
| 编号 | 实验值 | 计算值 | 相对误差 | 编号 | 实验值 | 计算值 | 相对误差 |
|---|---|---|---|---|---|---|---|
| 1,A | 1.345 2 | 1.345 2 | 0.000 0 | 12 | 1.374 0 | 1.388 6 | -0.010 7 |
| 2 | 1.346 1 | 1.348 3 | -0.001 7 | 13 | 1.375 7 | 1.388 9 | -0.009 6 |
| 3 | 1.346 8 | 1.350 2 | -0.002 5 | 14 | 1.379 8 | 1.394 3 | -0.010 5 |
| 4 | 1.347 5 | 1.350 4 | -0.002 1 | 15 | 1.383 2 | 1.398 1 | -0.010 8 |
| 5,E1 | 1.348 1 | 1.350 8 | -0.001 8 | 16 | 1.406 9 | 1.407 3 | -0.000 3 |
| 6 | 1.351 1 | 1.354 5 | -0.002 5 | 17 | 1.410 2 | 1.409 4 | 0.000 6 |
| 7 | 1.355 9 | 1.361 9 | -0.004 4 | 18,E2 | 1.409 6 | 1.409 0 | 0.000 4 |
| 8 | 1.358 7 | 1.365 5 | -0.005 0 | 19 | 1.410 5 | 1.407 9 | 0.001 8 |
| 9 | 1.360 5 | 1.369 5 | -0.006 6 | 20 | 1.409 9 | 1.409 5 | 0.000 2 |
| 10 | 1.368 2 | 1.382 5 | -0.010 5 | 21,B | 1.409 5 | 1.409 5 | 0.000 0 |
| 11 | 1.369 7 | 1.384 3 | -0.010 6 |
| [1] | 卢靖, 王颖松, 蒋育澄 , 等. 金属铯的生物化学研究进展[J]. 稀有金属, 2006,30(5):682-687. |
| [2] | 黄万抚, 李新冬 . 铯的用途与提取分离技术研究现状[J]. 稀有金属与硬质合金, 2003,31(3):18-20. |
| [3] | 董普, 肖荣阁 . 铯盐应用及铯(碱金属)矿产资源评价[J]. 中国矿业, 2005,14(2):30-34. |
| [4] | Merbach A, Gonella J . Contribution à l′étude du système quaternaire KCl-RbCl-H2O:Ⅰ.Les isothermes de 25° des systèmes ternaires limites[J]. Helvetica Chimica Acta, 1969,52(1):69-76. |
| [5] | Merbach A, Gonella J . Contribution à l′étude du système quaternaire KCl-RbCl-CsCl-H2O:Ⅱ.L′isotherme de 25 ℃[J]. Helvetica Chi-mica Acta, 1971,54(5):1380-1389. |
| [6] | Yu X, Zeng Y, Yao H , et al. Metastable phase equilibria in the aque-ous ternary systems KCl+MgCl2+H2O and KCl+RbCl+H2O at 298.15 K[J]. Journal of Chemical & Engineering Data, 2011,56(8):3384-3391. |
| [7] | 宝阿敏, 钱志强, 郑红 , 等. 铷、铯的分离提取方法及其研究进展[J]. 应用化工, 2017,46(7):1377-1382. |
| [8] | 汪傲, 赵元艺, 许虹 , 等. 青藏高原盐湖资源特点概述[J]. 盐湖研究, 2016,24(3):24-29. |
| [9] | 郑绵平, 侯献华 . 青海盐湖资源综合利用与可持续发展战略[J]. 科技导报, 2017,35(12):11-13. |
| [10] | 冯凯, 孙亚猛, 袁梦 , 等. 以盐湖高含泥提钾尾矿制备微孔分子筛的研究[J]. 无机盐工业, 2018,50(5):76-80. |
| [11] | 宋彭生, 李武, 孙柏 , 等. 盐湖资源开发利用进展[J]. 无机化学学报, 2011,27(5):801-815. |
| [12] | 牛自得, 程芳琴 . 水盐体系相图及其应用[M]. 天津: 天津大学出版社, 2002. |
| [13] | 伍倩, 乜贞, 卜令忠 , 等. 三元体系Li+,K+//CO32--H2O 298.15 K稳定相平衡研究[J]. 无机盐工业, 2018,50(6):28-31. |
| [14] | Hu M C, Zhang W J, Li S N , et al. Thermodynamic investigation of a ternary mixed electrolyte(CsCl/MgCl2/H2O) system using electro-motive force measurement at 298.15 K[J]. Journal of Chemical & Engineering Data 2009,54(7):2023-2027. |
| [15] |
Balarew Chr, Christov Chr, Valyashko Vl , et al. Thermodynamics of formation of carnallite type double salts[J]. Journal of Solution Chemistry, 1993,22(2):173-181.
doi: 10.1007/BF00650683 |
| [16] |
Li Y, Song P, Xia S , et al. Effects of Pitzer mixing parameters on the solubility prediction of HCl-CsCl-H2O system at 25 ℃[J]. Russian Journal of Inorganic Chemistry, 2006,51(10):1659-1663.
doi: 10.1134/S0036023606100238 |
| [17] |
Hu B, Song P, Li Y , et al. Solubility prediction in the ternary systems NaCl-RbCl-H2O,KCl-CsCl-H2O and KBr-CsBr-H2O at 25 ℃ using the ion-interaction model[J]. Calphad-Computer Coupling of Phase Diagrams and Thermochemistry, 2007,31(4):541-544.
doi: 10.1016/j.calphad.2007.03.002 |
| [18] | Guo L, Wang Y, Tu L , et al. Thermodynamics and phase equilibrium of the system CsCl-MgCl2-H2O at 298.15 K[J]. Journal of Chemi-cal & Engineering Data, 2017,62(4):1397-1402. |
| [19] | Zhang Y M, Ma L C, Liu C L , et al. Phase diagram and research for the ternary system of NaCl-CsCl-H2O at 50 ℃[J]. Advanced Ma-terials Research, 2013, 706-708:193-196. |
| [20] | 保英莲, 孙海霞, 曹红翠 , 等. Pitzer混合参数对CsCl-Cs2SO4-H2O体系25 ℃时溶解度预测的影响[J]. 盐业与化工, 2012,41(10):17-19. |
| [21] | Hu B, Song P, Li Y . Solubility prediction of CsCl-Cs2SO4-H2O system at 298.15 K using the ion-interaction model[J]. The Open Thermo-dynamics Journal, 2007,1:1-3. |
| [22] | 中国科学院青海盐湖研究所分析室. 卤水和盐的分析方法[M]. 北京: 科学出版社, 1988. |
| [23] | 姚允斌, 解涛 . 物理化学手册[M]. 上海: 上海科学技术出版社, 1985. |
| [24] | 金利华 . 稀碱金属铯盐在脂肪醇水体系的等温相行为及热力学研究[D]. 西安:陕西师范大学, 2006. |
| [25] | 秦善 . 晶体学基础[M]. 北京: 北京大学出版社, 2004. |
| [26] | Gao Y, Li S, Zhai Q , et al. Phase diagrams and physicochemical pro-perties for the ternary system(CsCl+NaCl+H2O) at T=(298.15,308.15,and 318.15) K[J]. Journal of Chemical & Engineering Data, 2017,62(9):2533-2540. |
| [1] | Yang Jiamin,Zhu Jing,Hu Xue,Wu Qiang,Li Tianxiang. Determination and correlation for solid-liquid phase equilibrium of ternary KCl-NH4Cl-H2O and KH2PO4-NH4H2PO4-H2O systems at 283.15 K [J]. Inorganic Chemicals Industry, 2021, 53(1): 30-35. |
| [2] | Zhang Peng,Zhang Yu,Duan Jiangfei,Lin Xiaohua. Research on synthesis of barium sulfate by ion-exchange method [J]. Inorganic Chemicals Industry, 2020, 52(5): 40-44. |
| [3] | Liao Enxin,Chen Lifang,Zhang Zeya,Bai Fengxia. Experimental study on preparing potassium sulfate by sodium sulfate and potassium chloride [J]. Inorganic Chemicals Industry, 2020, 52(10): 106-109. |
| [4] | Chen Xia,Bai Fengxia,Shi Yapeng,Dong Sicheng,Liao Enxin,Li Ying,Miao Shulan. Experimental research of preparation of potassium sulfate from high-salt wastewater in coal chemical industry by double decomposition method [J]. Inorganic Chemicals Industry, 2019, 51(6): 57-61. |
| [5] | Zhang Pingjun,Yu Shujuan. Online analysis on effect of ethanol and ultrasonic wave on crystal nucleus of potassium sulfate [J]. Inorganic Chemicals Industry, 2019, 51(6): 65-68. |
| [6] | CHEN Wen-Xing, TIAN Juan, ZHOU Chang-Ping. Preparation technology of potassium sulfate with insoluble potassium contained shale [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(7): 42-. |
| [7] | HUA Zong-Wei, ZHONG Hong, WANG Shuai, JIANG Chong-Wen. Research progress of production technology of potassium sulfate [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(4): 1-. |
| [8] | LU Qi-Fu, SANG Shi-Hua, ZHANG Hai, LI Ting. Research of phase equilibrium in ternary system KBr-K2SO4-H2O at 373 K [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(2): 13-. |
| [9] | ZHU Zhi-Qing, WANG Ke. Preparation of potassium sulphate from separation of mixed solution of potassium and sodium salts with improved methanol salting-out method [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(7): 18-. |
| [10] | CHEN Ju-Ling, XIAO Jing-Bo, WANG Wei-Dong. Study on joint-production technology of magnesium compound and potassium sulfate from dolomite [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(7): 35-. |
| [11] | ZHANG Yu-Cui. Discussion on water balance for production of potassium sulfate by Glauber′s salt method [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(3): 36-. |
| [12] | DONG Zhan-Neng, HAO Shi-Yong, DENG Lai. Study on one-stage process of producing potassium sulfate fertilizer from phosphogypsum [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(11): 52-. |
| [13] | LIU Bin, YU Shao-Ming, ZHANG Ying, CHENG Lei-Lei, CHA Cai-Cun. Study on comprehensive recovery and utilization of waste vanadium catalyst [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(11): 57-. |
| [14] | LI Chun-Li, ZHANG Zhi-Qiang, GUAN Yun-Shan, XING Ying, WANG Juan, ZHANG Ying-Zhi, HE Quan-Sheng. Study on preparation of potassium dihydrogen phosphate with calcium hydrogen phosphate Ⅰ [J]. INORGANICCHEMICALSINDUSTRY, 2011, 43(7): 48-. |
| [15] | Yang Lihong. Application of stirring equipment in production of potassium sulfate by mirabilite process [J]. INORGANICCHEMICALSINDUSTRY, 2010, 0(9): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|