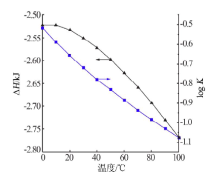
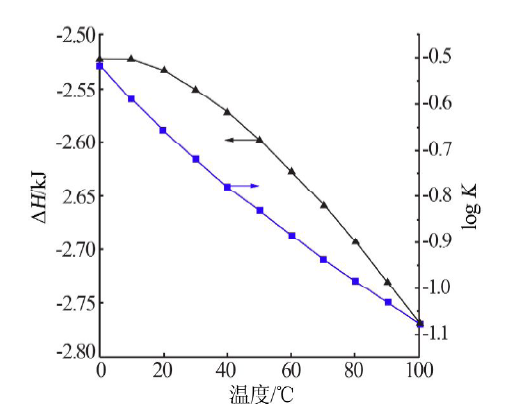
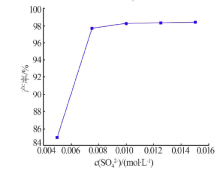
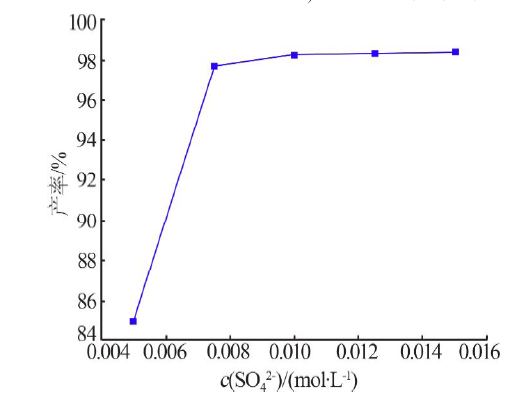
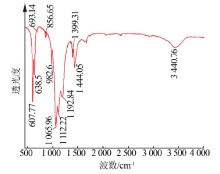
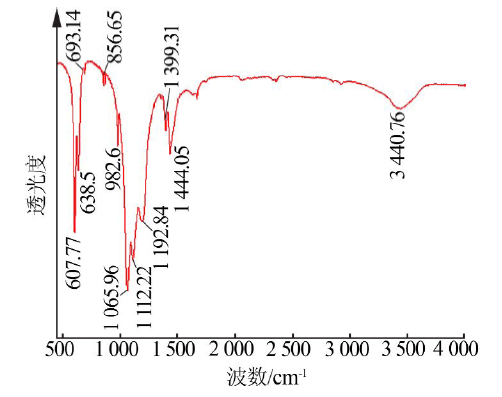
| [1] |
祁琪, 孙青, 张俭 , 等. 超细沉淀硫酸钡的制备及研究进展[J]. 无机盐工业, 2018,50(5):15-20.
|
| [2] |
Ke Z, Bai Y P . Improve the gas barrier property of PET film with montmorillonite by in situ interla-yer polymerization[J]. Materials Letters, 2005,59(27):3348-3351.
|
| [3] |
曹荣 . 功能性微细硫酸钡的制备研究[D]. 天津:河北工业大学, 2012.
|
| [4] |
Meagher M J, Leone B, Turnbull T L . Dextran-encapsulated barium sulfate nanoparticles prepared for aqueous dispersion as an X-ray contrast agent[J]. J.Nanopart Res., 2013,15:2146.Doi: 10.1007/s11051-013-2146-8.
|
| [5] |
尚方毓, 胡昉, 苏小红 . 浅谈国内沉淀硫酸钡生产现状及发展趋势[J]. 无机盐工业, 2015,47(1):1-4.
|
| [6] |
阎振宗, 董晓菁, 段亚宏 . 碳酸钡芒硝转化法生产硫酸钡[J]. 河北化工, 2013,36(3):56-57.
|
| [7] |
尚久芳, 操时杰, 辛无名 , 等. 兰式化学手册[D]. 北京: 科学出版社, 1991.
|
| [8] |
Zhao Chenhao, Liu Fenglin, Kang Wenpei , et al. An ion-exchange approach to the crystal design of barium sulfate in the presence of ionic surfactants[J]. Cryst.Growth Des., 2011,11:2084-2090.
|
| [9] |
祁琪, 孙青, 张俭 , 等. EDTA络合-球磨法制备超细棒状BaSO4[J]. 高校化学工程学报, 2019,33(3):732-737.
|
| [10] |
Haley L V, Mattioli T A, Wiles DR . Micro-Raman study of sulphate coprecipitated with potassium ions[J]. Journal of Raman Spectro-scopy, 1987(18):101-104.
|
| [11] |
樊先平, 洪樟连, 翁文剑 . 无机非金属材料科学基础[M]. 杭州: 浙江大学出版社, 2004.
|
| [12] |
Liu Yuejiao, Guo Xiaoyao, Yang Suohe , et al. Controllable preparation of uniform micronsized barium sulfate spheres[J]. Crystal Research & Technology, 2018,53(6).Doi: 10.1002/crat.201700212.
|
| [13] |
李丽匣, 韩跃新, 陶世杰 . 碳酸钙晶须表面改性研究[J]. 化工矿物与加工, 2008,37(5):4-8.
|
| [14] |
闻辂, 梁婉雪, 章正刚 . 矿物红外光谱学[M]. 重庆: 重庆大学出版社, 1988.
|
| [15] |
张明, 李新海, 胡启阳 , 等. EDTA络合法合成硫酸钡微粒[J]. 中国有色金属学报, 2009,19(8):1512-1516.
|
 ),Duan Jiangfei,Lin Xiaohua
),Duan Jiangfei,Lin Xiaohua