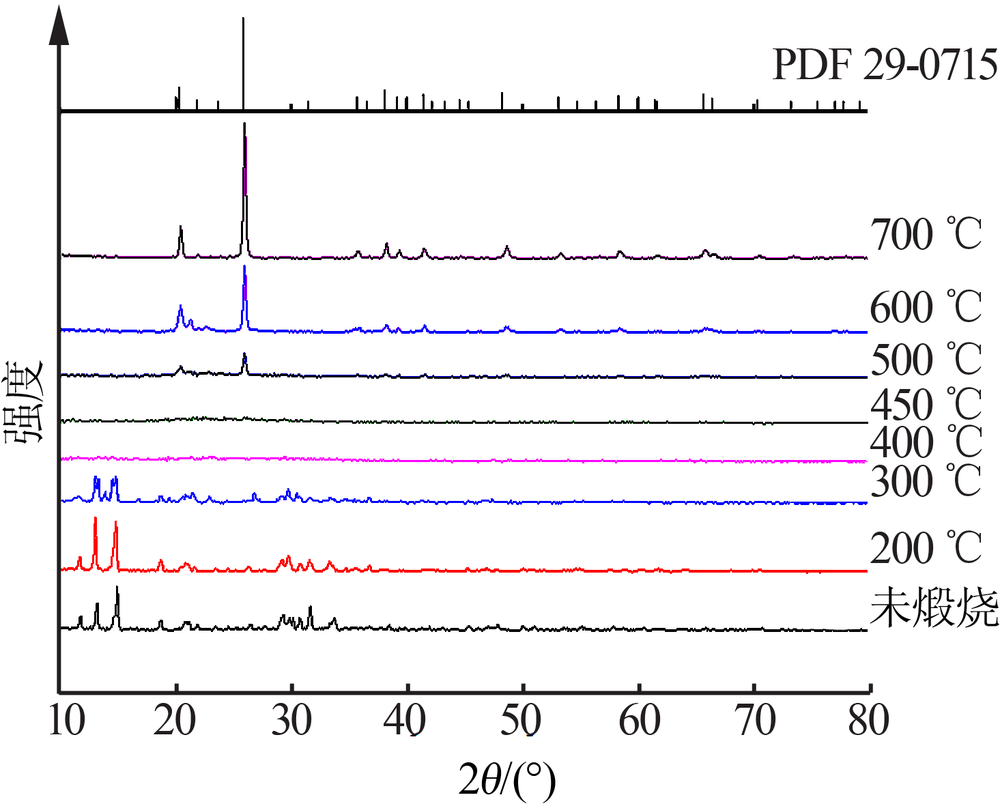
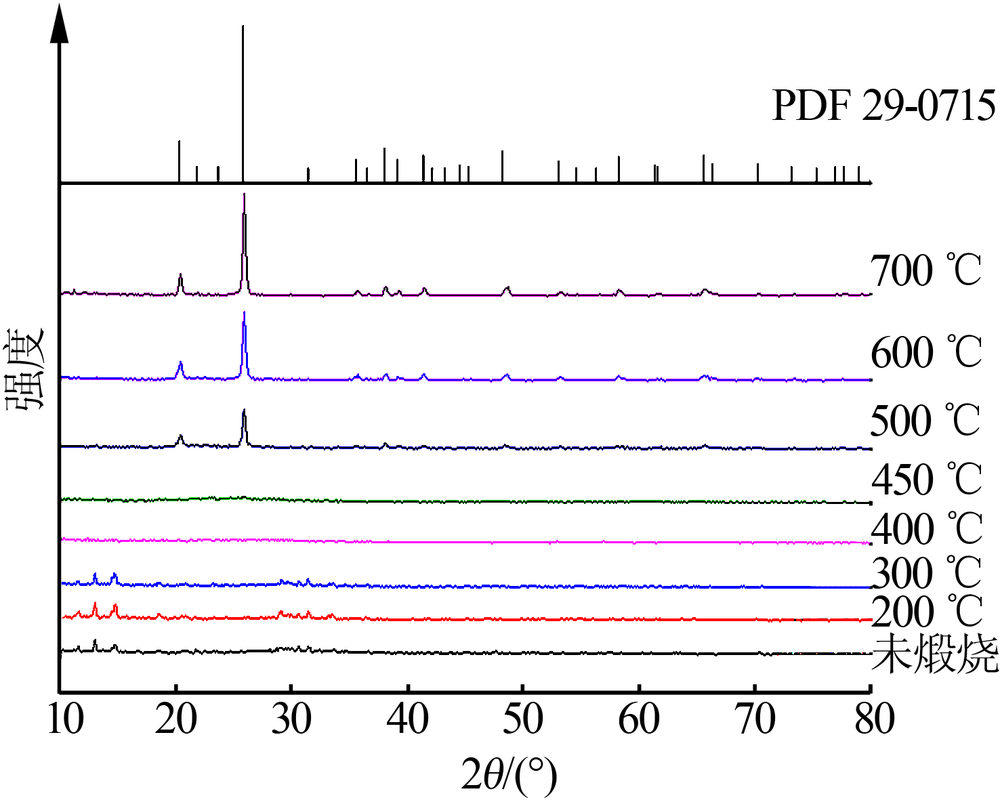
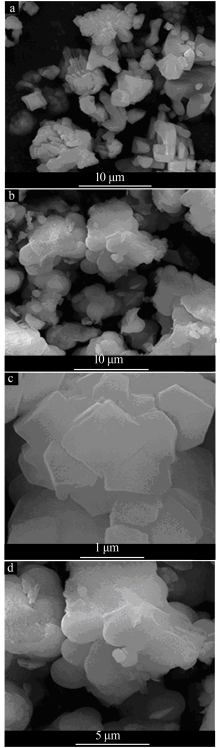
Inorganic Chemicals Industry ›› 2019, Vol. 51 ›› Issue (5): 53-56.
• Research & Development • Previous Articles Next Articles
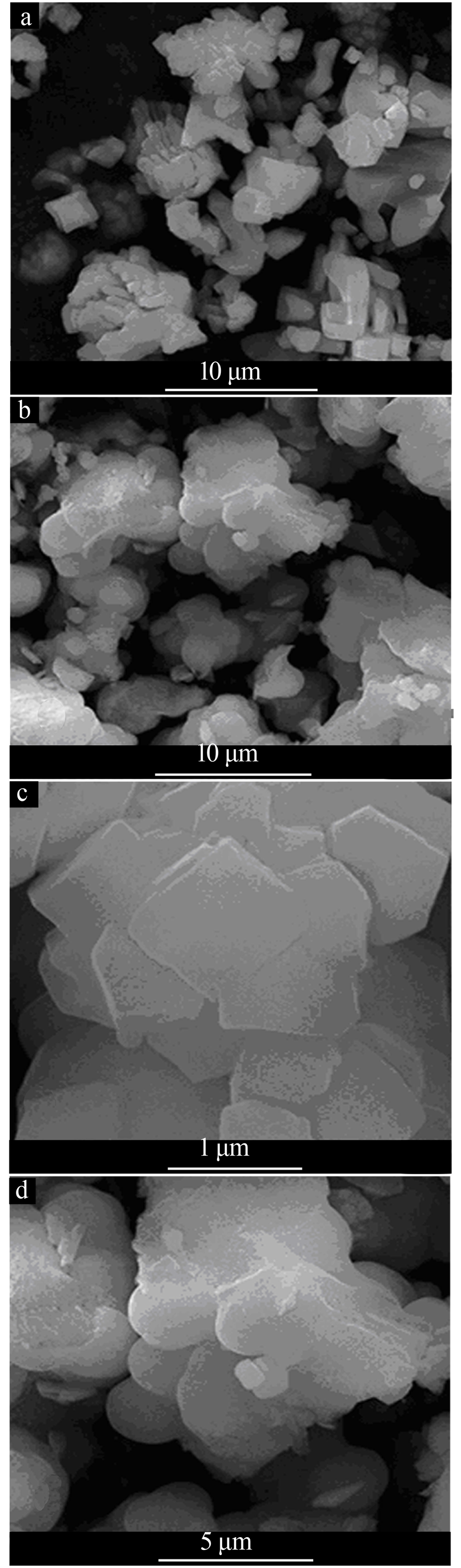
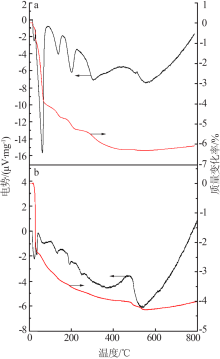
Preparation and characterization of spheniscidite
Mao Qiqi1,Liu Chuanxin1,Dong Wenjuan1,Zhang Ximan1,Chen Hongyu1,Wu Suwen2
- 1. College of Chemistry and Pharmaceutical Engineering,Taishan Medical College,Taian 271016,China
2. Shandong Huanglan Weiye New Energy Technology Co.,Ltd.
-
Received:2018-11-27Online:2019-05-10Published:2020-04-28
CLC Number:
Cite this article
Mao Qiqi,Liu Chuanxin,Dong Wenjuan,Zhang Ximan,Chen Hongyu,Wu Suwen. Preparation and characterization of spheniscidite[J]. Inorganic Chemicals Industry, 2019, 51(5): 53-56.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] |
Padhi A K, Nanjundaswamy K S, Goodenough J B . Phospho-olivines as positive-electrode materials for rechargeable lithium batteries[J]. Journal of the Electrochemical Society, 1997,144(4):1188-1194.
doi: 10.1149/1.1837571 |
| [2] |
Lee J, Kumar P, Lee G , et al. Electrochemical performance of surfactant-processed LiFePO4 as a cathode material for lithium-ion rechargeable batteries[J]. Ionics, 2013,19(2):371-378.
doi: 10.1007/s11581-012-0830-9 pmid: 19354236 |
| [3] |
Zhang W J . Structure and performance of LiFePO4 cathode materials:A review[J]. Journal of Power Sources, 2011,196(6):2962-2970.
doi: 10.1016/j.jpowsour.2010.11.113 pmid: 30875978 |
| [4] | Jugovic D, Uskokovic D . A review of recent developments in the synjournal procedures of lithium iron phosphate powders[J]. Journal of Power Sources, 2009,190(2):538-544. |
| [5] | 郑典模, 陈骏驰, 叶焕英 , 等. 正交法优化沉淀法制备超细磷酸铁工艺[J]. 电源技术, 2014,38(5):815-818. |
| [6] | Son D, Kim E, Kim T G , et al. Nanoparticle iron-phosphate anode material for Li-ion battery[J]. Applied Physics Letters, 2004,85(24):5875-5877. |
| [7] |
Lin Y, Wu J B, Chen W P . Enhanced electrochemical performance of LiFePO4/C prepared by sol-gel synjournal with dry ball-milling[J]. Ionics, 2013,19(2):227-234.
doi: 10.1007/s11581-012-0735-7 |
| [8] | Wang Q, Deng S X, Wang H , et al. Hydrothermal synjournal of hierarchical LiFePO4 microspheres for lithium ion battery[J]. Chemiform, 2013,553(14):69-74. |
| [9] | Novikova S A, Yaroslavtsev A B . Cathode materials based on olivine lithium-ion batteries[J]. Rev.Adv.Mater.Sci., 2017,49:129-139. |
| [10] | 易均辉 . 磷酸铁和磷酸铁锂的制备,表征及电化学性能的研究[D]: 南宁:广西大学, 2010. |
| [11] | Calderón C A, Thomas J E, Lener G , et al. Electrochemical comparison of LiFePO4 synthesized by a solid-state method using either microwave heating or a tube furnace[J]. Journal of Applied Elec-trochemistry, 2017,47(10):1179-1188. |
| [12] | 韩梦莹 . 电池级磷酸铁的制备工艺研究[D]: 郑州:郑州大学, 2014. |
| [13] |
Masquelier C, Reale P, Wurm C , et al. Hydrated iron phosphates FePO4·nH2O and Fe4(P2O7)3·nH2O as 3V positive electrodes in rechargeable lithium batteries[J]. Journal of the Electrochemical Society, 2002,149(8):A1037-A1044.
doi: 10.1149/1.1489686 |
| [14] | 赖桂棠, 李大光, 李军 , 等. 共沉淀法制备球形磷酸亚铁铵[J]. 化工新型材料, 2007,35(11):38-39,42. |
| [15] | 田晓珍, 廖森, 吴昆 , 等. 水合磷酸铁铵的低热固相合成及其对乙酸丁酯的催化作用[J]. 广西科学, 2008,15(2):166-169. |
| [16] | Trobajo C, Espina A, Jaimez E , et al. Hydrothermal synjournal of iron(Ⅲ) phosphates in the presence of urea[J]. Journal of the Chemical Society,Dalton Transactions, 2000(5):787-790. |
| [17] | Sugiura S, Sugiura S . Nakamura T.Fuel cell including separator with outer ends placed inward of fluid passages formed in frame:US,9660276[P].2017-05-23. |
| [18] |
Choudhury A, Natarajan S . A synthetic iron phosphate mineral,spheniscidite,[NH 4] +[Fe2(OH)(H2O)(PO4)2]·H2O,exhibiting reversible dehydration [J]. Journal of Chemical Sciences, 1999,111(5):627-637.
doi: 10.1007/BF02869118 |
| [19] |
Wu K P, Liu D W, Tang Y . Self-assembly of red-blood-cell-like (NH4)[Fe2(OH)(PO4)2]·2H2O architectures from 2D nanoplates by sonochemical method[J]. Ultrasonics Sonochemistry, 2018,40:832-836.
doi: 10.1016/j.ultsonch.2017.08.036 pmid: 28946492 |
| [20] | Liu X B., Liu G B., Wang Y , et al. Influence of pH value on the properties of NH4Fe2(OH) -(PO4)2·2H2O precursor and LiFePO4/C composite [J]. Journal of Electronic Materials, 2015,44(3):1008-1014. |
| [1] | Liu Peiwen,Dong Peng,Meng Qi,Yang Xuan,Zhou Siyuan. Research development of solid phase regeneration of cathode material of spent lithium iron phosphate batteries [J]. Inorganic Chemicals Industry, 2020, 52(9): 6-8. |
| [2] | Wang Guoping,Xu Xuhui,Li Gang,Qiu Xuhui,Liu Zhengwei. Identification and analysis of fouling composition of ammonium chloride evaporator [J]. Inorganic Chemicals Industry, 2020, 52(8): 81-83. |
| [3] | Yan Bo,Xie Tian,Zhu Huacheng,Yang Fengming,Wang Fengxia,Chen Yong. Study on process of synthesizing ammonium polyphosphate by microwave heating [J]. Inorganic Chemicals Industry, 2020, 52(7): 42-45. |
| [4] | Li Xiangguo,Guo Miao,Yao Huimin,Lü Jing. Research on hydrothermal synthesis and photoluminescence mechanism of rhombus CeO2 [J]. Inorganic Chemicals Industry, 2020, 52(6): 30-35. |
| [5] | Guo Ju,Jia Shuangzhu. Study on the one-step hydrothermal synthesis of LiFePO4 and its properties [J]. Inorganic Chemicals Industry, 2020, 52(6): 36-40. |
| [6] | Wu Kang,Li Jun,Chen Ming. Synthesis and characterization of 3D nano/micro spherical iron phosphate in ethanol-water system [J]. Inorganic Chemicals Industry, 2020, 52(6): 41-45. |
| [7] | Guo Ju,Jia Shuangzhu. Study of the method for the preparation of spherical-like and low sulfur FePO4 [J]. Inorganic Chemicals Industry, 2020, 52(5): 31-34. |
| [8] | Chen Yong,Yang Sanke,Xie Tian,Tao Shaocheng,Long Qinglan,Liu Xu. Research on preparation process of water-soluble ammonium polyphosphate with high polymerization rate [J]. Inorganic Chemicals Industry, 2020, 52(5): 50-52. |
| [9] | Hu Zengbin,Li Zongze,Li Shaohua,Cun Jie,Xu Yonge. ICP-AES determination of phosphorous content in iron phosphate for battery materials [J]. Inorganic Chemicals Industry, 2020, 52(5): 78-80. |
| [10] | Wang Jiatai,Zhao Duan,Ma Lianhua,Zhang Caihong. Research progress of LiFePO4 cathode materials for Li-ion battery [J]. Inorganic Chemicals Industry, 2020, 52(4): 18-22. |
| [11] | Liao Xiaoting,Li Jun,Chen Ming. Process study on preparation of industrial grade ammonium dihydrogen phosphate from urea phosphate mother liquor [J]. Inorganic Chemicals Industry, 2020, 52(4): 79-83. |
| [12] | Yin Haijiao,Xia Junjun,Quan Xiaoyu,Peng Tao,Tang Hao. Study on operation characteristics of mechanical flash cooling crystallization technology for titanium solution in summer [J]. Inorganic Chemicals Industry, 2020, 52(4): 93-95. |
| [13] | Wang Baoming,Lü Zhonghao,Li Huiyong,Liu Yong,Hua Quanxian,Tang Jianwei. Research progress on preparation of urea phosphate with wet-process phosphoric acid [J]. Inorganic Chemicals Industry, 2020, 52(11): 1-5. |
| [14] | Liu Ying. Synthesis of magnesium ferrite photocatalyst and degradation activity of various dyes [J]. Inorganic Chemicals Industry, 2020, 52(11): 103-107. |
| [15] | Huang Zhenxu,He Huanhuan,Jia Panpan,Chen Tiwei,Wei Shiqian. Synthesis of graphene by hydrothermal method and its electrocatalytic property on ascorbic acid [J]. Inorganic Chemicals Industry, 2020, 52(11): 29-32. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|