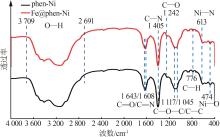
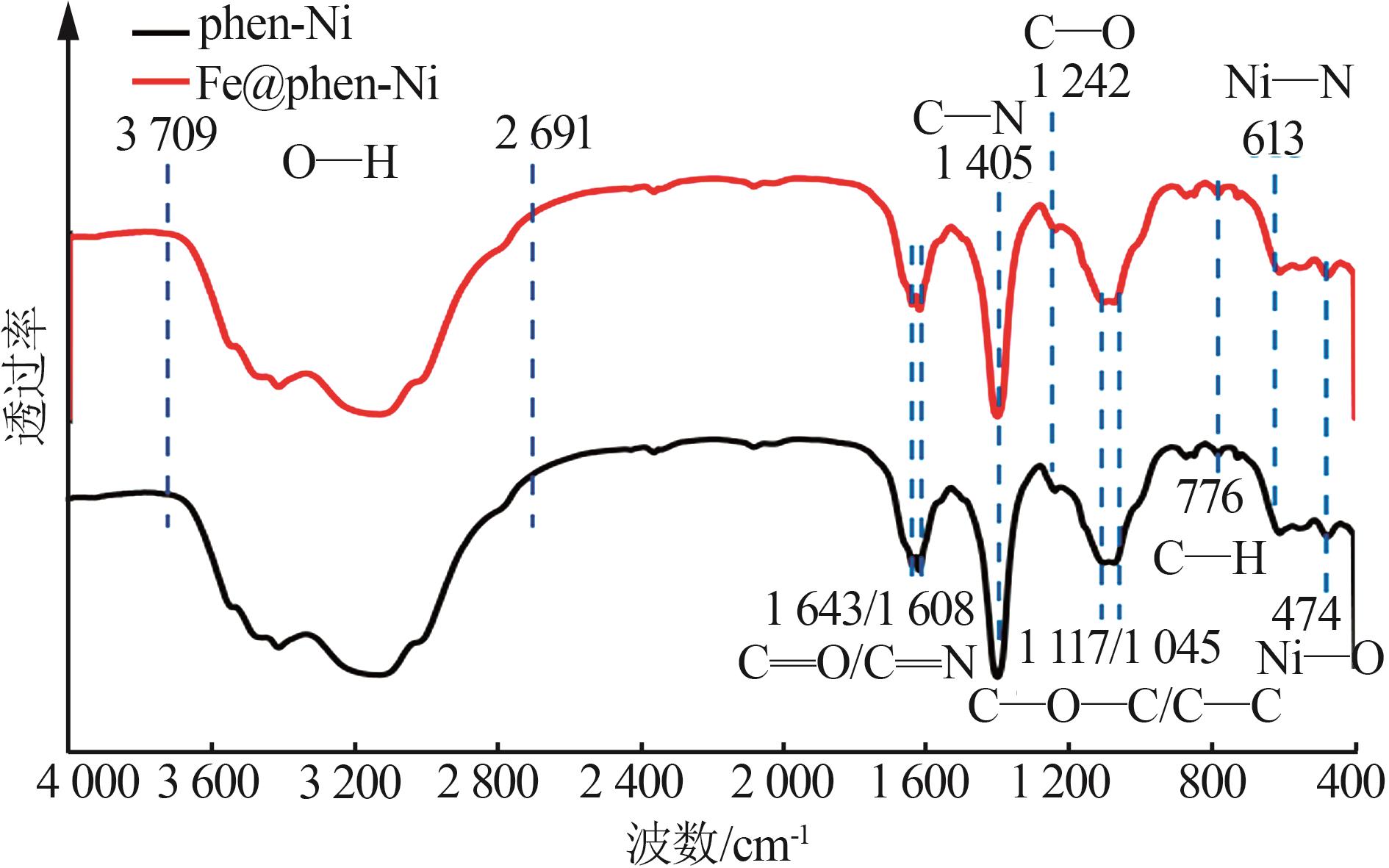
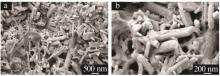
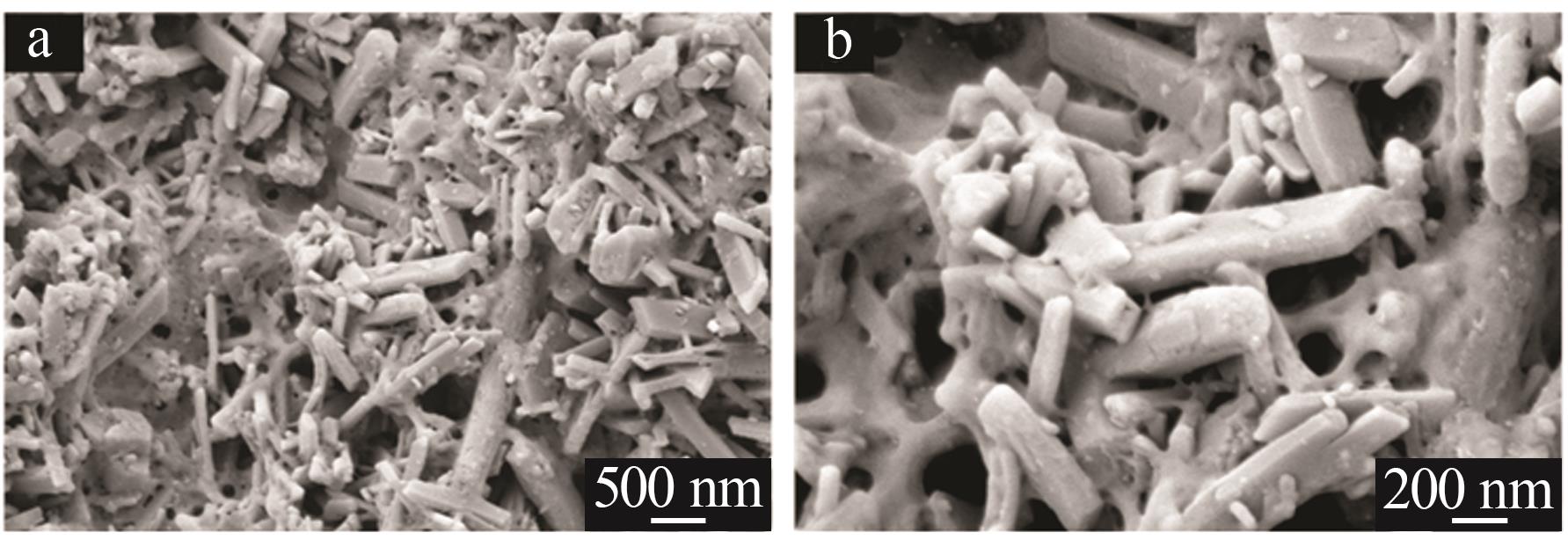
| 1 |
谢士礼.钴基金属有机框架材料的制备及其电催化析氧性能研究[D].大连:大连理工大学,2019.
|
|
XIE Shili.Fabrication of Co-based metal-organic frameworks for electrocatalytic oxygen evolution[D].Dalian:Dalian University of Technology,2019.
|
| 2 |
ZHANG Bowei, LUI Y H, GAUR A P S,et al.Hierarchical FeNiP@ultrathin carbon nanoflakes as alkaline oxygen evolution and acidic hydrogen evolution catalyst for efficient water electrolysis and organic decomposition[J].ACS Applied Materials & Interfaces,2018,10(10):8739-8748.
|
| 3 |
WU Zhihan, WANG Zhiqiang, GENG Fengxia.Radially aligned hierarchical nickel/nickel-iron(oxy)hydroxide nanotubes for efficient electrocatalytic water splitting[J].ACS Applied Materials & Interfaces,2018,10(10):8585-8593.
|
| 4 |
ZHUANG Wenchang, DU Minglin, LU Xinhua,et al.Fe doping mo-difying electronic structure of NiSe2 for boosting electrocatalytic oxygen evolution reaction[J].Ionics,2023,29(3):1069-1076.
|
| 5 |
陈小明,张杰鹏.金属-有机框架材料[M].北京:化学工业出版社,2017.
|
| 6 |
SANATI S, MORSALI A, GARCÍA H.First-row transition metal-based materials derived from bimetallic metal-organic frameworks as highly efficient electrocatalysts for electrochemical water splitting[J].Energy & Environmental Science,2022,15(8):3119-3151.
|
| 7 |
DONG Anrui, CHEN Dandan, LI Qipeng,et al.Metal-organic fra-meworks for greenhouse gas applications[J].Small,2023,19(10):2201550.
|
| 8 |
FÉREY G.Hybrid porous solids:Past,present,future[J].Chemical Society Reviews,2008,37(1):191-214.
|
| 9 |
沈威,王思楠,梁雪梅,等.纳米MOFs及其衍生物在超级电容器中的研究进展[J].无机盐工业,2021,53(6):79-86.
|
|
SHEN Wei, WANG Sinan, LIANG Xuemei,et al.Research progress of nano MOFs and their derivatives for supercapacitors[J].Inorganic Chemicals Industry,2021,53(6):79-86.
|
| 10 |
叶谢维伊,郑志平,匡勤.二维ZIF-L衍生的叶片状Fe-NC材料的制备及其氧还原催化性能研究[J].无机盐工业,2021,53(6):1-7.
|
|
YE Xieweiyi, ZHENG Zhiping, KUANG Qin.Preparation of 2D leaf-like Fe-NC materials derived from ZIF-L and study on their oxygen reduction catalytic performance[J].Inorganic Che-micals Industry,2021,53(6):1-7.
|
| 11 |
LIU Ming, KONG Lingjun, WANG Xuemin,et al.Engineering bimetal synergistic electrocatalysts based on metal-organic frameworks for efficient oxygen evolution[J].Small,2019,15(45):1903410.
|
| 12 |
ZHAO Shenlong, TAN Chunhui, HE Chunting,et al.Structural transformation of highly active metal-organic framework electrocatalysts during the oxygen evolution reaction[J].Nature Energy,2020,5(11):881-890.
|
| 13 |
ZHOU Hongcai, LONG J R, YAGHI O M.Introduction to metal-organic frameworks[J].Chemical Reviews,2012,112(2):673- 674.
|
| 14 |
DOLGOPOLOVA E A, BRANDT A J, EJEGBAVWO O A,et al.Electronic properties of bimetallic metal-organic frameworks (MOFs):Tailoring the density of electronic states through MOF modularity[J].Journal of the American Chemical Society,2017,139(14):5201-5209.
|
| 15 |
ZHAO Ming, GUO Taolian, QIAN Wei,et al.Fe-incorporated cobalt-based metal-organic framework ultrathin nanosheets for electrocatalytic oxygen evolution[J].Chemical Engineering Journal,2021,422:130055.
|
| 16 |
WANG Qijun, WEI Feifei, MANOJ D,et al. In situ growth of Fe(Ⅱ)-MOF-74 nanoarrays on nickel foam as an efficient electrocatalytic electrode for water oxidation:A mechanistic study on valence engineering[J].Chemical Communications,2019,55(75):11307-11310.
|
| 17 |
HAN Mengyi, ZHANG Xiaowei, GAO Hongyi,et al. In situ semi-sacrificial template-assisted growth of ultrathin metal-organic framework nanosheets for electrocatalytic oxygen evolution[J].Chemical Engineering Journal,2021,426:131348.
|
| 18 |
TROTOCHAUD L, YOUNG S L, RANNEY J K,et al.Nickel-iron oxyhydroxide oxygen-evolution electrocatalysts:The role of intentional and incidental iron incorporation[J].Journal of the American Chemical Society,2014,136(18):6744-6753.
|
| 19 |
HAN Zhengbo, CHENG Xiaoning, CHEN Xiaoming.Effect of the size of aromatic chelate ligands on the frameworks of metal dicarboxylate polymers:From helical chains to 2-D networks[J].Crystal Growth & Design,2005,5(2):695-700.
|
| 20 |
TIAN Junwu, WU Yapan, LI Yongshuang,et al.Integration of semiconductor oxide and a microporous(3,10)-connected Co6-based metal-organic framework for enhanced oxygen evolution reaction[J].Inorganic Chemistry,2019,58(9):5837-5843.
|
| 21 |
WANG Xiaoli, DONG Longzhang, QIAO Man,et al.Exploring the performance improvement of the oxygen evolution reaction in a stable bimetal-organic framework system[J].Angewandte Chemie,2018,57(31):9660-9664.
|
| 22 |
XIA Baoyu, YAN Ya, LI Nan,et al.A metal-organic framework-derived bifunctional oxygen electrocatalyst[J].Nature Energy,2016,1:15006.
|
| 23 |
DOU Shuo, DONG C L, HU Zhe,et al.Atomic-scale CoO x species in metal-organic frameworks for oxygen evolution reaction[J].Advanced Functional Materials,2017,27(36):1702546.
|
 ), FAN Wenjuan2, CHANG Hui2, HUANG Haiping1, JIANG Zhiqiang2(
), FAN Wenjuan2, CHANG Hui2, HUANG Haiping1, JIANG Zhiqiang2( )
)