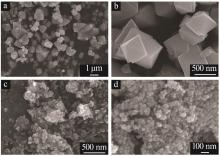
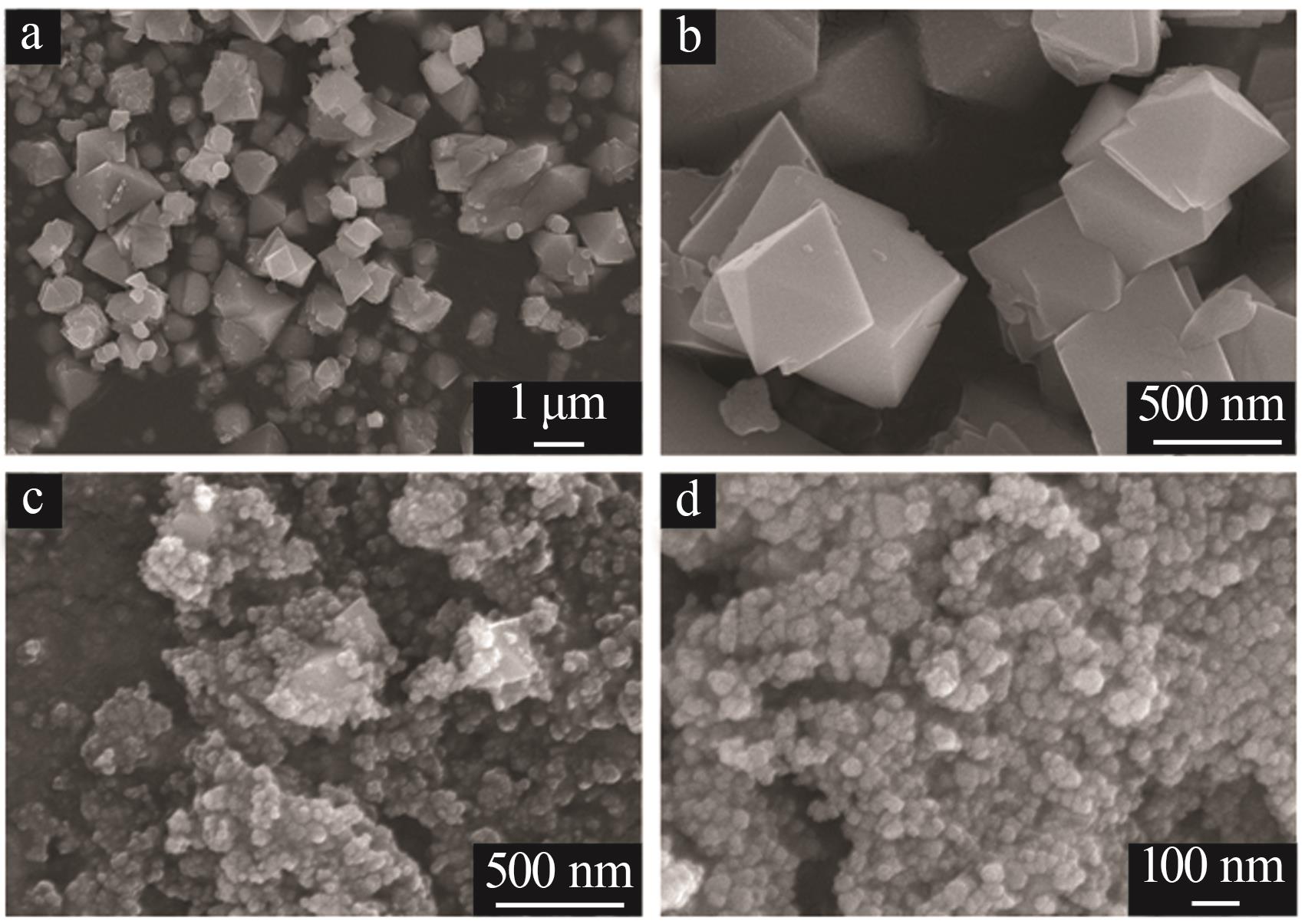
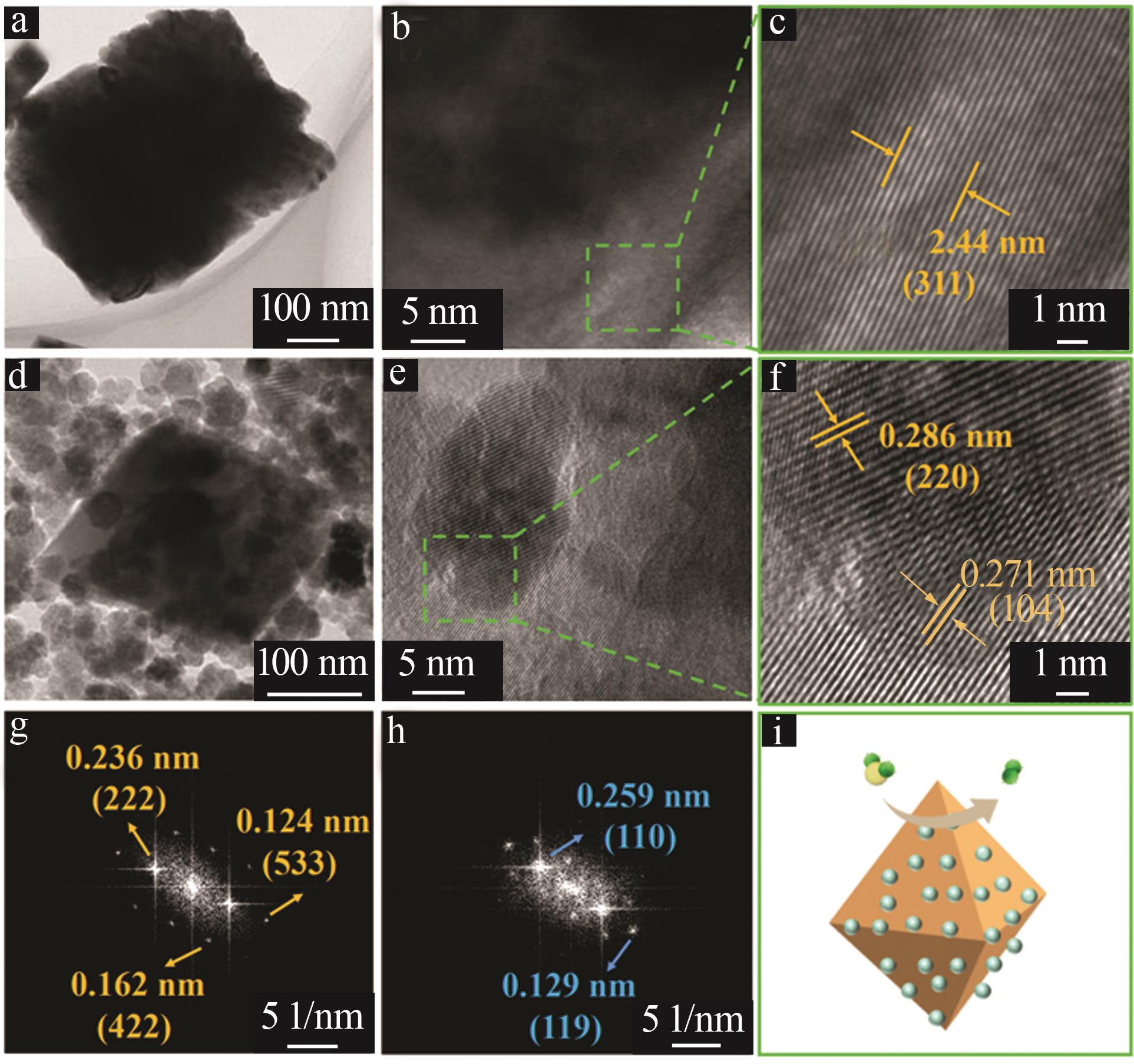
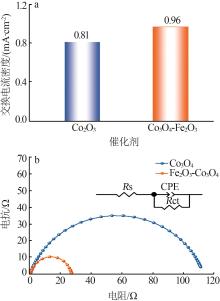
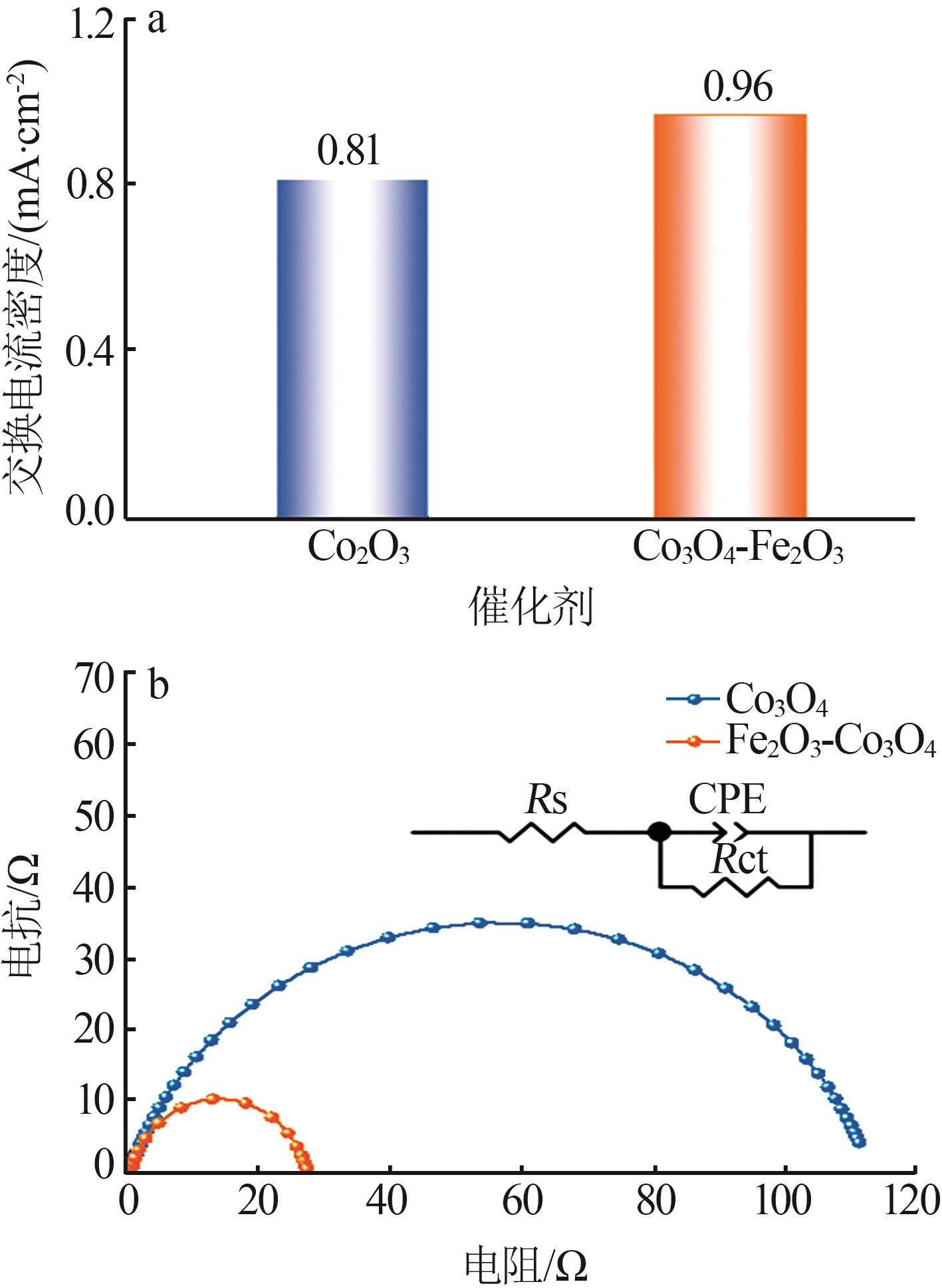
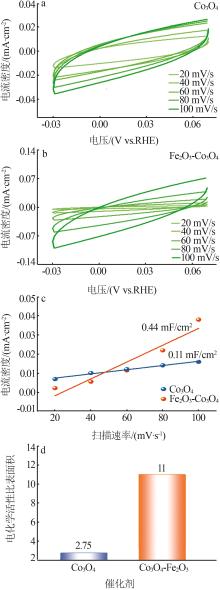
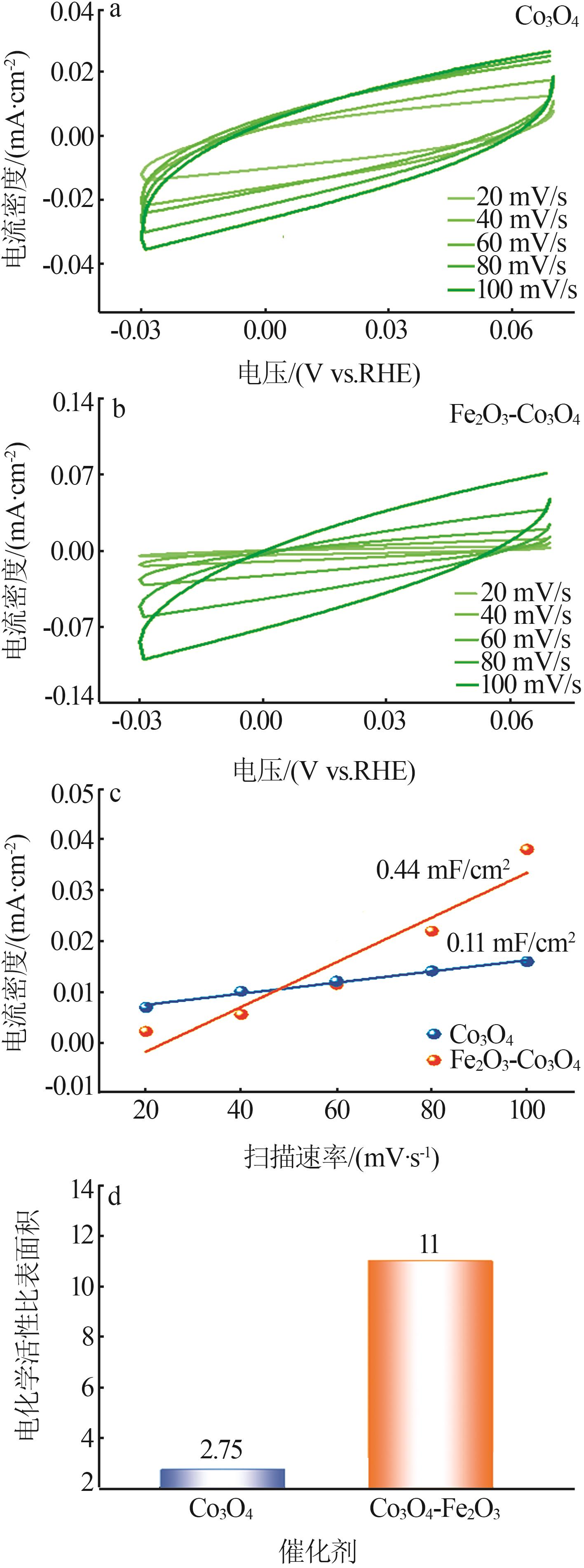
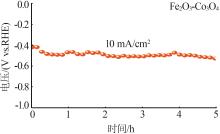
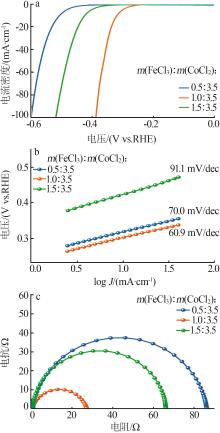
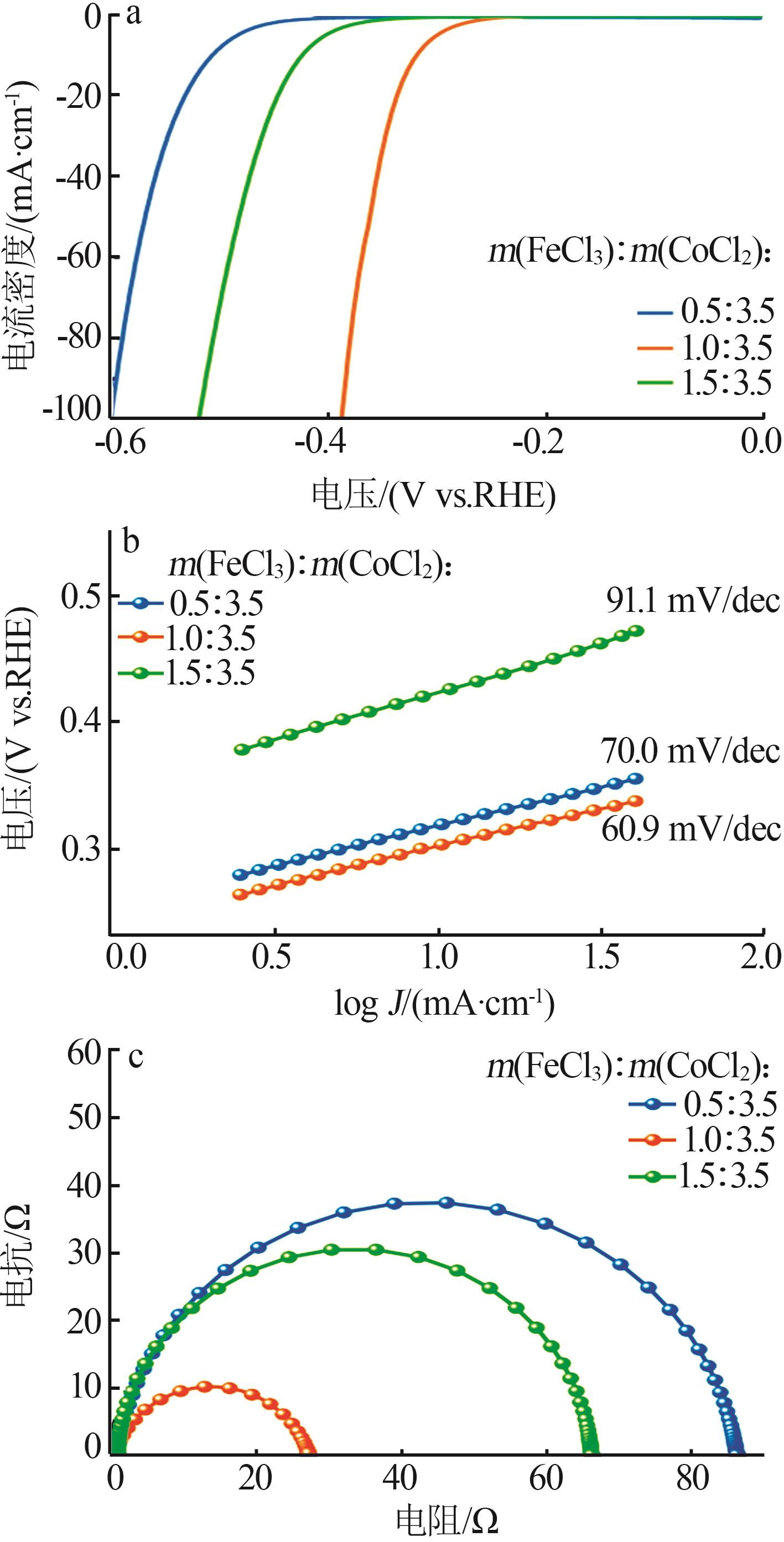
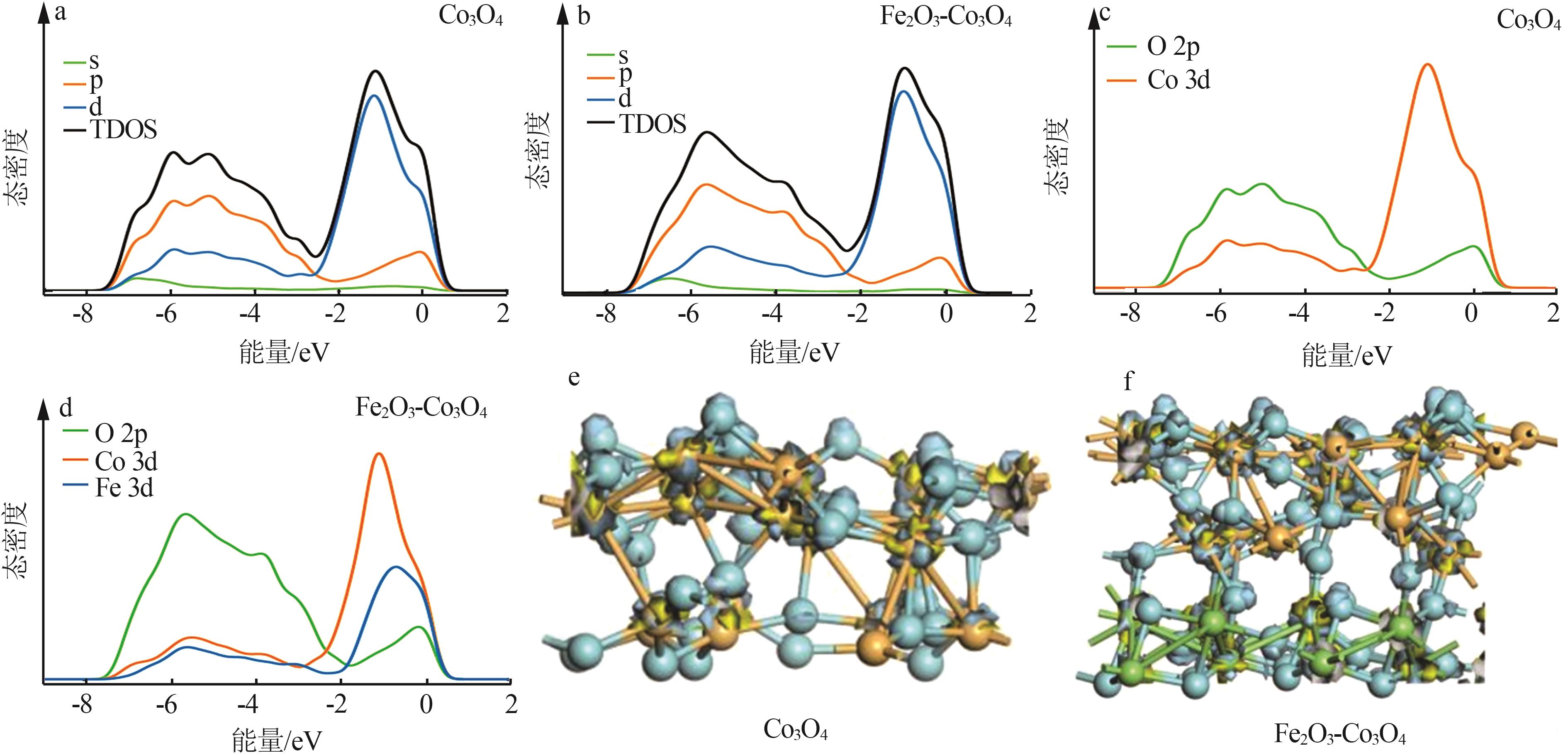
| 1 |
BORETTI A.The hydrogen economy is complementary and synergetic to the electric economy[J].International Journal of Hydrogen Energy,2021,46(78):38959-38963.
|
| 2 |
DILLMAN K J, HEINONEN J.A‘just’hydrogen economy:A normative energy justice assessment of the hydrogen economy[J].Renewable and Sustainable Energy Reviews,2022,167:112648.
|
| 3 |
ZHANG Jiachen, CHEN Guangbo, LIU Qicheng,et al.Competitive adsorption:Reducing the poisoning effect of adsorbed hydroxyl on Ru single-atom site with SnO2 for efficient hydrogen evolution[J].Angewandte Chemie International Edition,2022,61(39):e202209486.
|
| 4 |
CHEN Guangbo, WANG Tao, ZHANG Jian,et al.Accelerated hydrogen evolution kinetics on NiFe-layered double hydroxide electrocatalysts by tailoring water dissociation active sites[J].Advanced Materials,2018,30(10):1706279.
|
| 5 |
YUAN Ling, LIU Song, XU Shichen,et al.Modulation of Volmer step for efficient alkaline water splitting implemented by titanium oxide promoting surface reconstruction of cobalt carbonate hydroxide[J].Nano Energy,2021,82:105732.
|
| 6 |
ZHANG Junyu, LIAN Jianshe, JIANG Qing,et al.Boosting the OER/ORR/HER activity of Ru-doped Ni/Co oxides heterostructure[J].Chemical Engineering Journal,2022,439:135634.
|
| 7 |
MARTINI B K, MAIA G.Using a combination of Co,Mo,and Pt oxides along with graphene nanoribbon and MoSe2 as efficient catalysts for OER and HER[J].Electrochimica Acta,2021,391:138907.
|
| 8 |
LI Dan, CHEN Xufang, LV Yaozha,et al.An effective hybrid electrocatalyst for the alkaline HER:Highly dispersed Pt sites immobilized by a functionalized NiRu-hydroxide[J].Applied Catalysis B:Environmental,2020,269:118824.
|
| 9 |
THIRUMAL V, YUVAKKUMAR R, SENTHIL KUMAR P,et al.Morphology investigation on direct growth ultra-long CNTs by chemical vapour deposition method for high performance HER applications[J].Fuel,2022,330:125532.
|
| 10 |
LALWANI S, ALNAHYAN M, ZAABI A AL,et al.Advances in interfacial engineering and their role in heterostructure formation for HER applications in wider pH[J].ACS Applied Energy Materials,2022,5(12):14571-14592.
|
| 11 |
WANG Tingting, WANG Pengyan, ZANG Wenjie,et al.Nanoframes of Co3O4-Mo2N heterointerfaces enable high-performance bifunctionality toward both electrocatalytic HER and OER[J].Advanced Functional Materials,2022,32(7):2107382.
|
| 12 |
LI Xintong, LIU Yizhe, SUN Qidi,et al.Surface engineered CoP/Co3O4 heterojunction for high-performance bi-functional water splitting electro-catalysis[J].Nanoscale,2021,13(47):20281-20288.
|
| 13 |
夏昱.熔融盐法制备氧化铁纳米催化剂消除挥发性有机物的研究[D].北京:北京工业大学,2018.
|
|
XIA Yu.Molten-salt derived iron oxide catalysts and their performance for the removal of volatile organic compounds[D].Beijing:Beijing University of Technology,2018.
|
| 14 |
HU Zhimi, XIAO Xu, JIN Huanyu,et al.Rapid mass production of two-dimensional metal oxides and hydroxides via the molten salts method[J].Nature Communications,2017,8:15630.
|
| 15 |
MA Guoliang, SHAO Hui, XU Jin,et al.Li-ion storage properties of two-dimensional titanium-carbide synthesized via fast one-pot method in air atmosphere[J].Nature Communications,2021,12:5085.
|
| 16 |
LAMB J, JARVIS K, MANTHIRAM A.Molten-salt synthesis of O3‐Type layered oxide single crystal cathodes with controlled morphology towards long-life sodium-ion batteries[J].Small,2022,18(43):2106927.
|
| 17 |
GUO Shuoshuo, WU Yongmeng, WANG Changhong,et al.Electrocatalytic hydrogenation of quinolines with water over a fluorine-modified cobalt catalyst[J].Nature Communications,2022,13:5297.
|
| 18 |
HOU Xianbiao, JIANG Tianyuan, XU Xiujuan,et al.Coupling of NiFe-based metal-organic framework nanosheet arrays with embedded Fe-Ni3S2 clusters as efficient bifunctional electrocatalysts for overall water splitting[J].Chinese Journal of Structural Chemistry,2022,41(7):80-86.
|
| 19 |
HAN Zishan, CHOI C, HONG Song,et al.Activated TiO2 with tun->ed vacancy for efficient electrochemical nitrogen reduction[J].Applied Catalysis B:Environmental,2019,257:117896.
|
| 20 |
WALTER M G, WARREN E L, MCKONE J R,et al.Solar water splitting cells[J].Chemical Reviews,2010,110(11):6446-6473.
|
| 21 |
YAN Dafeng, CHEN Ru, XIAO Zhaohui,et al.Engineering the electronic structure of Co3O4 by carbon-doping for efficient overall water splitting[J].Electrochimica Acta,2019,303:316-322.
|
| 22 |
AFTAB U, TAHIRA A, SAMO A H,et al.Mixed CoS2@Co3O4 composite material:An efficient nonprecious electrocatalyst for hydrogen evolution reaction[J].International Journal of Hydrogen Energy,2020,45(27):13805-13813.
|
| 23 |
TAHIRA A, IBUPOTO Z H, MONTECCHI M,et al.Role of cobalt precursors in the synthesis of Co3O4 hierarchical nanostructures toward the development of cobalt-based functional electrocatalysts for bifunctional water splitting in alkaline and acidic media[J].Journal of the Chinese Chemical Society,2022,69(4):681-691.
|
| 24 |
BHUVANENDRAN N, CHOI M G, KIM D,et al.Improved trifunctional electrocatalytic performance of integrated Co3O4 spinel oxide morphologies with abundant oxygen vacancies for oxygen reduction and water-splitting reactions[J].Journal of Alloys and Compounds,2023,935:168079.
|
| 25 |
TRAN-PHU T, CHATTI M, LEVERETT J,et al.Understanding the role of(W,Mo,Sb) dopants in the catalyst evolution and activity enhancement of Co3O4 during water electrolysis via in situ spectroelectrochemical techniques[J].Small,2023,19(25):2208074.
|
| 26 |
GUO Kailu, WANG Yantao, HUANG Junfeng,et al. In situ activated Co3- x Ni x O4 as a highly active and ultrastable electrocatalyst for hydrogen generation[J].ACS Catalysts,2021,11(13):8174-8182.
|
 ), WANG Yinbin2, WEI Qiang1(
), WANG Yinbin2, WEI Qiang1( )
)