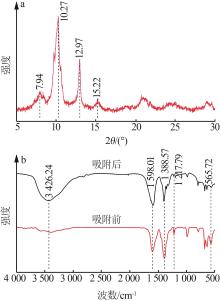
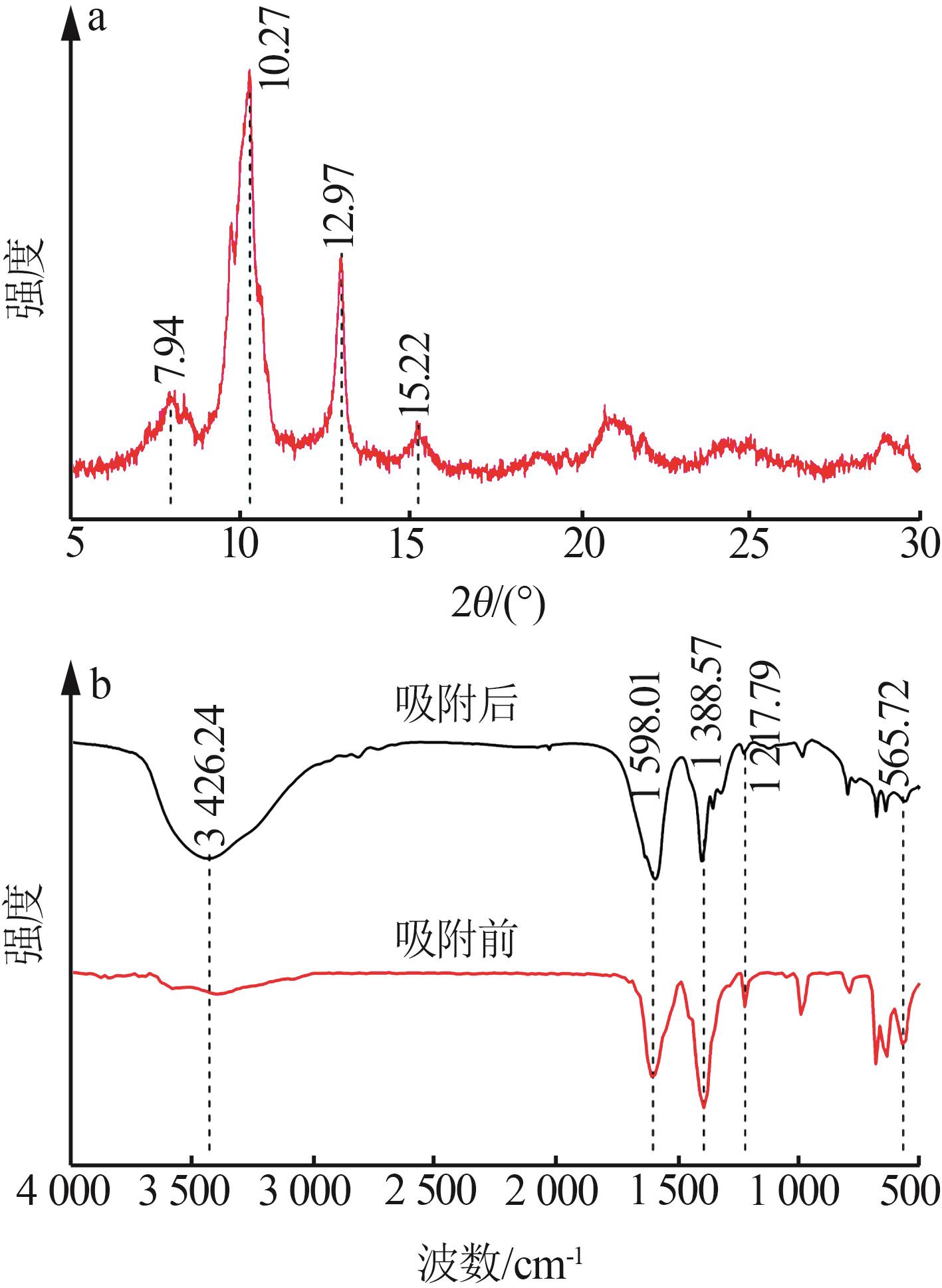
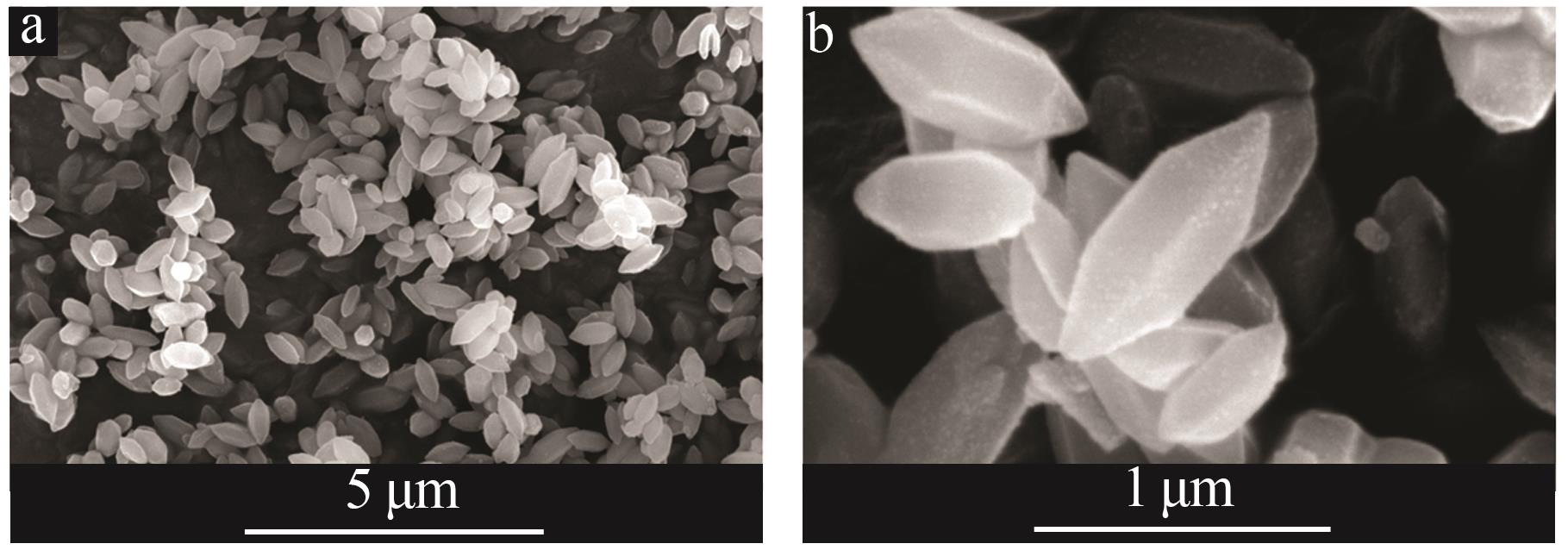
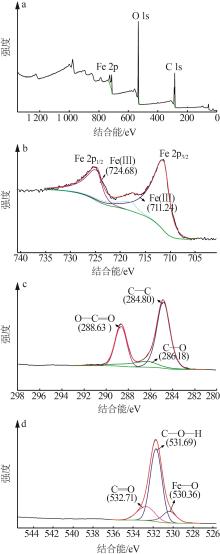
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (2): 111-120.doi: 10.19964/j.issn.1006-4990.2023-0221
• Environment·Health·Safety • Previous Articles Next Articles
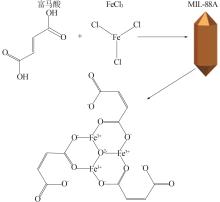
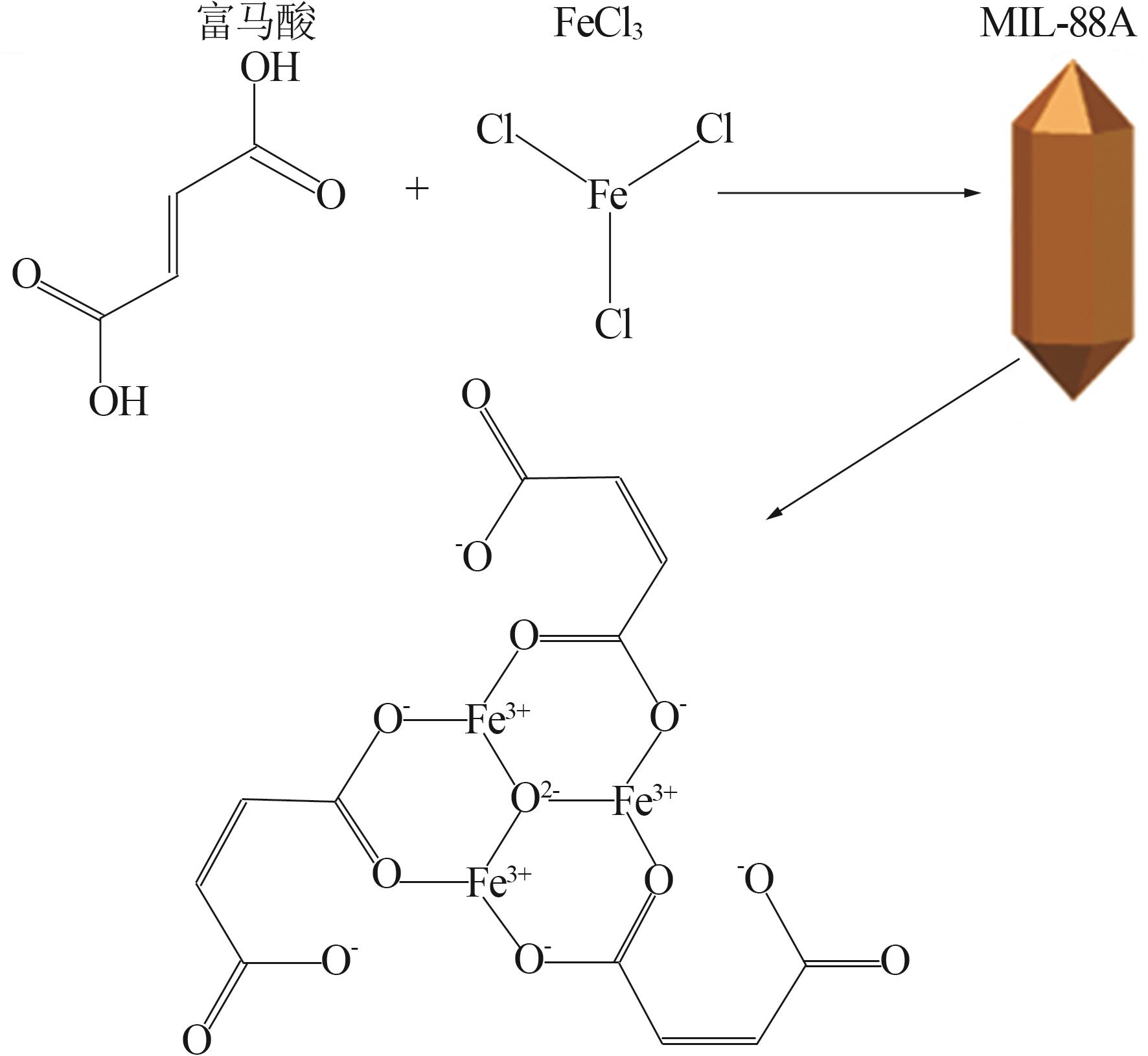
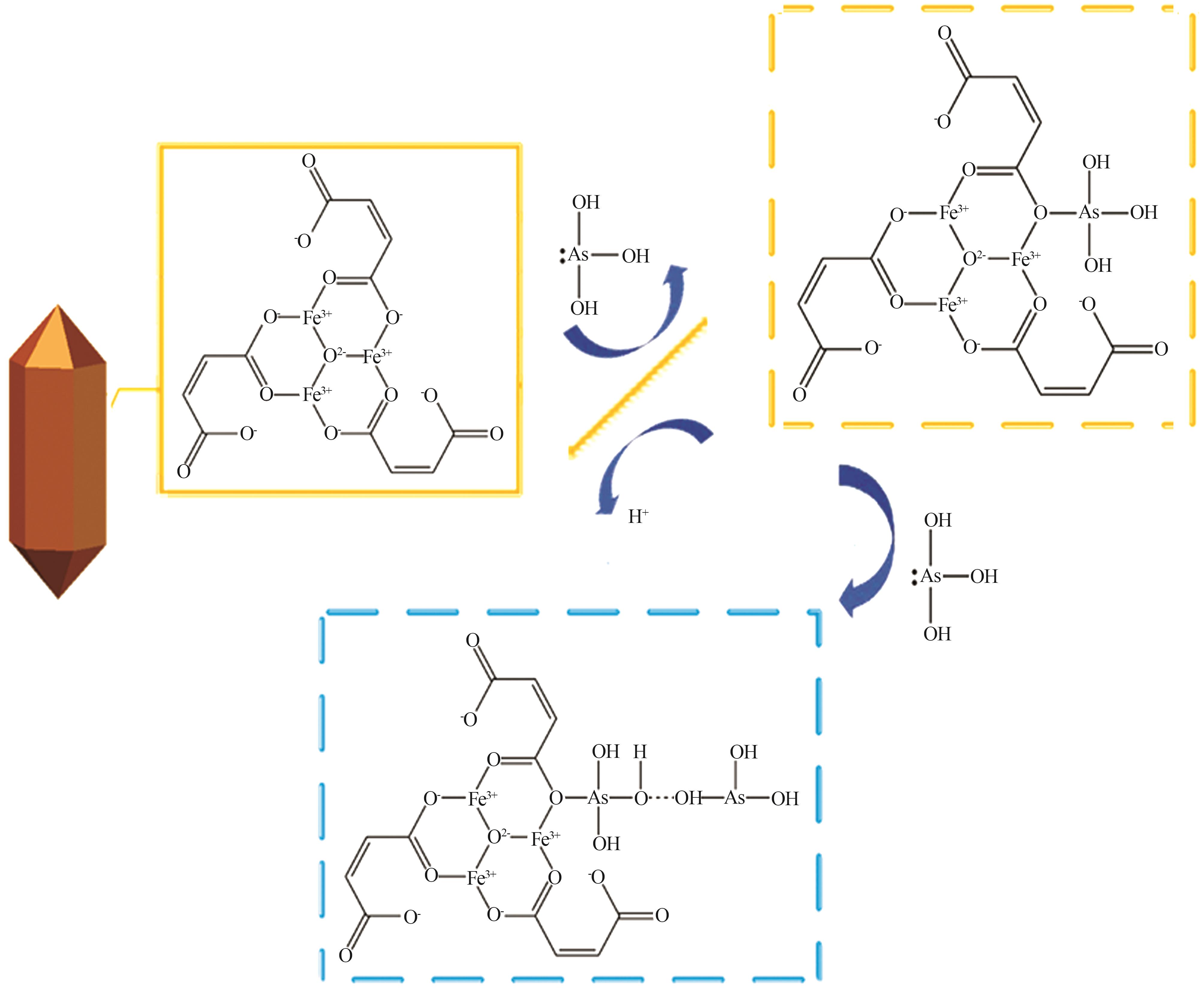
Research on preparation of iron-based organic metal-organic framework at room temperature for adsorption of trivalent arsenic
HE Yipeng( ), XIONG Chenxi, WANG Yiping, LI Jun, JIN Yang(
), XIONG Chenxi, WANG Yiping, LI Jun, JIN Yang( )
)
- School of Chemical Engineering,Sichuan University,Chengdu 610065,China
-
Received:2023-04-17Online:2024-02-10Published:2024-02-06 -
Contact:JIN Yang E-mail:1078878254@qq.com;jinyangyoung@126.com
CLC Number:
Cite this article
HE Yipeng, XIONG Chenxi, WANG Yiping, LI Jun, JIN Yang. Research on preparation of iron-based organic metal-organic framework at room temperature for adsorption of trivalent arsenic[J]. Inorganic Chemicals Industry, 2024, 56(2): 111-120.
share this article
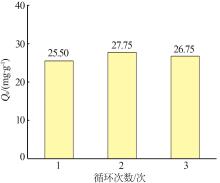
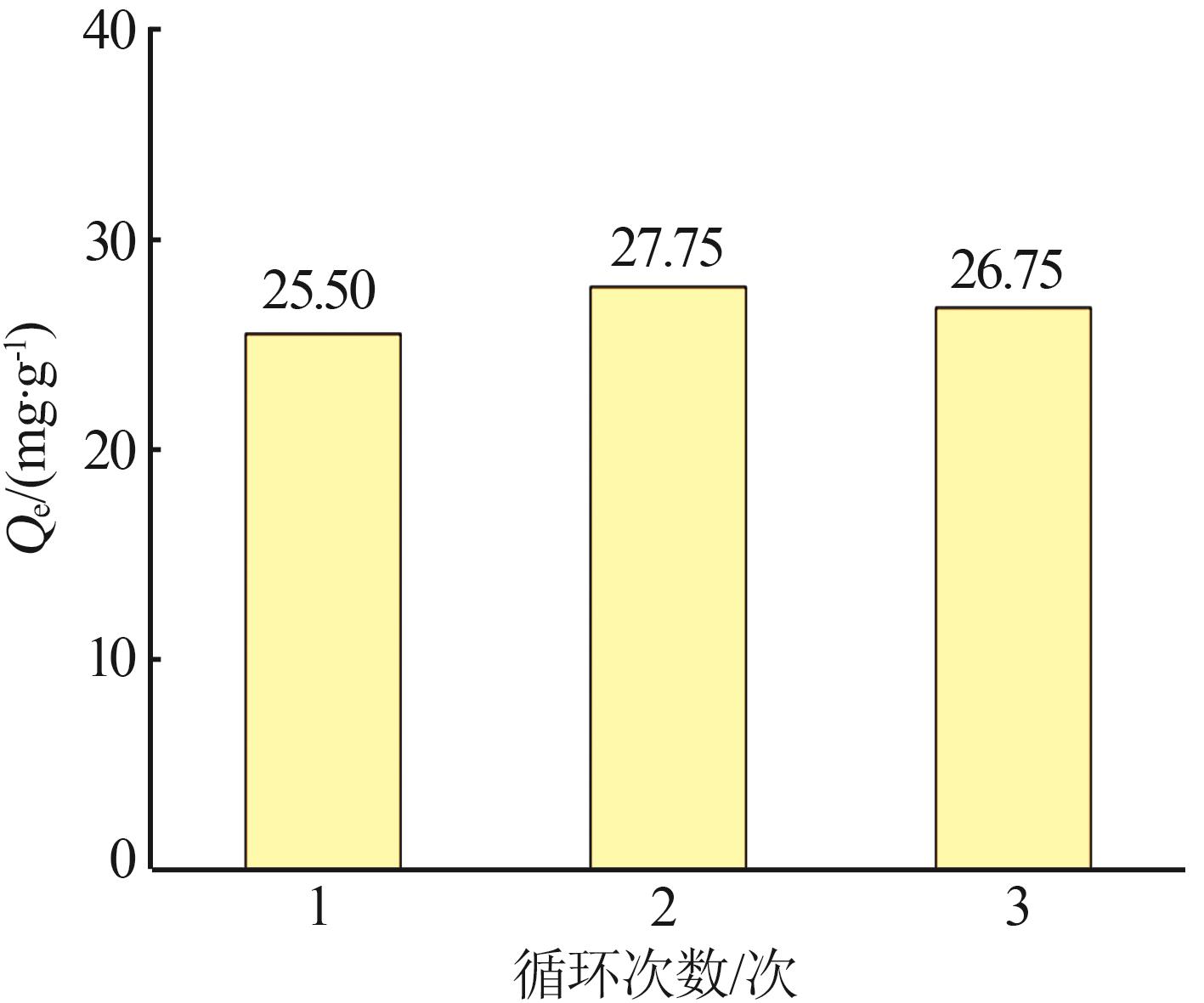
Table 1
Quasi-first-order and quasi-second-order kinetic model fitting parameters for adsorption of As(Ⅲ) by MIL-88A"
ρ0/ (mg·L-1) | 准一级动力学 | 准二级动力学 | 实验值 Qe/(mg·g-1) | |||||
|---|---|---|---|---|---|---|---|---|
| Qe1/(mg·g-1) | k1/(min-1) | R2 | Qe2/(mg·g-1) | k2/(g·mg-1·min-1) | R2 | |||
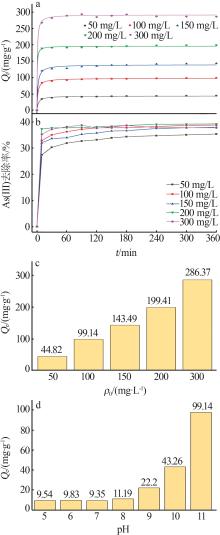
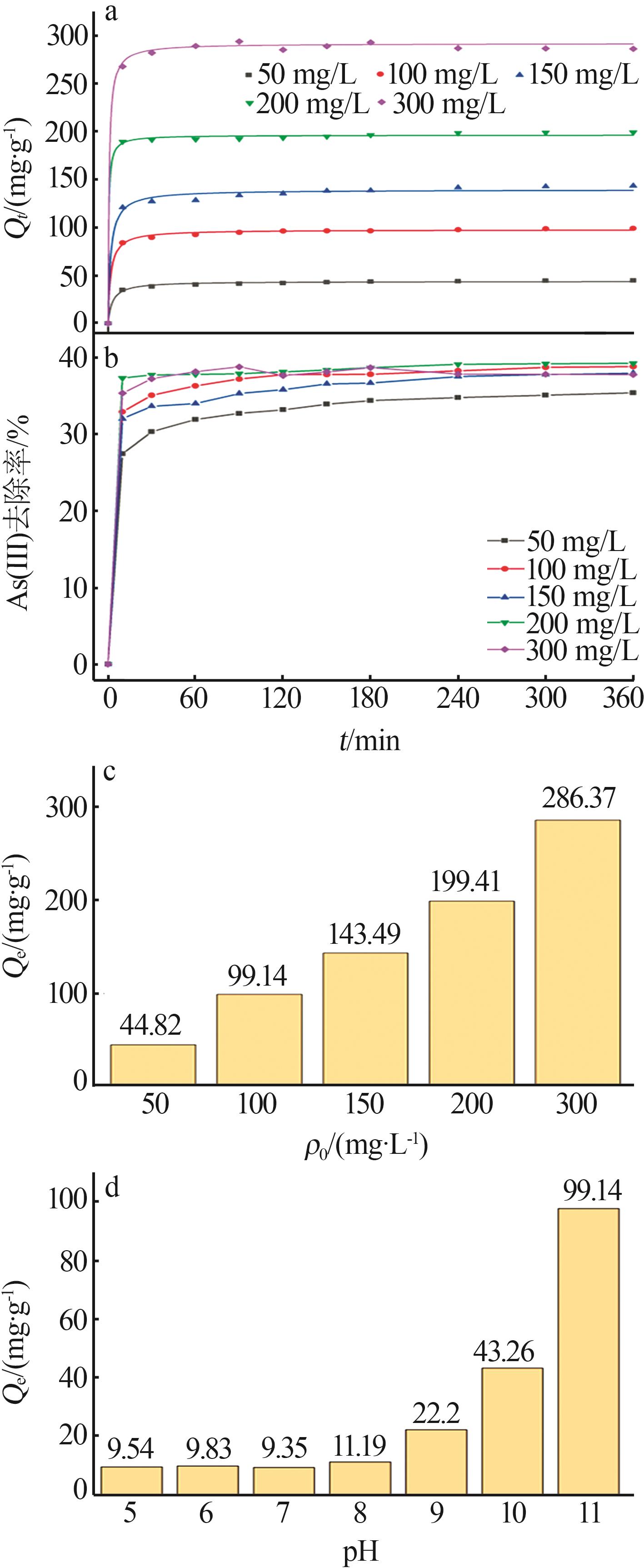
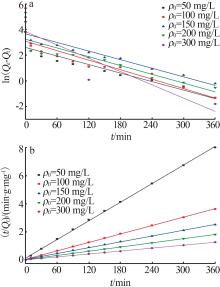
| 50 | 13.373 | 0.011 1 | 0.884 2 | 45.086 | 0.004 2 | 0.999 4 | 44.822 | |
| 100 | 21.782 | 0.012 3 | 0.847 5 | 99.407 | 0.003 2 | 0.999 8 | 99.137 | |
| 150 | 38.539 | 0.011 2 | 0.871 7 | 144.300 | 0.001 4 | 0.999 4 | 143.494 | |
| 200 | 25.475 | 0.011 4 | 0.731 2 | 200.000 | 0.002 5 | 0.999 8 | 199.413 | |
| 300 | 45.851 | 0.017 2 | 0.670 3 | 286.533 | 0.003 5 | 0.999 8 | 286.368 | |
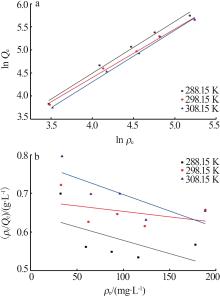
Table 2
Fitting parameters of isothermal linear model for adsorption of As(Ⅲ) by MIL-88A"
| T/K | Langmuir 模型 | Freundlich模型 | ||||||
|---|---|---|---|---|---|---|---|---|
Qm/ (103 mg·g-1) | KL/ (L·g-1) | R2 | KF/(mg1-1/n · L1/n ·g-1) | 1/n | R2 | |||
| 288.15 | -1.446 | -0.001 1 | 0.333 4 | 0.990 1 | 1.126 9 | 0.988 0 | ||
| 298.15 | -3.508 | -0.000 4 | 0.165 9 | 1.193 0 | 1.056 4 | 0.993 4 | ||
| 308.15 | -1.164 | -0.001 1 | 0.641 8 | 0.832 7 | 1.122 9 | 0.996 6 | ||
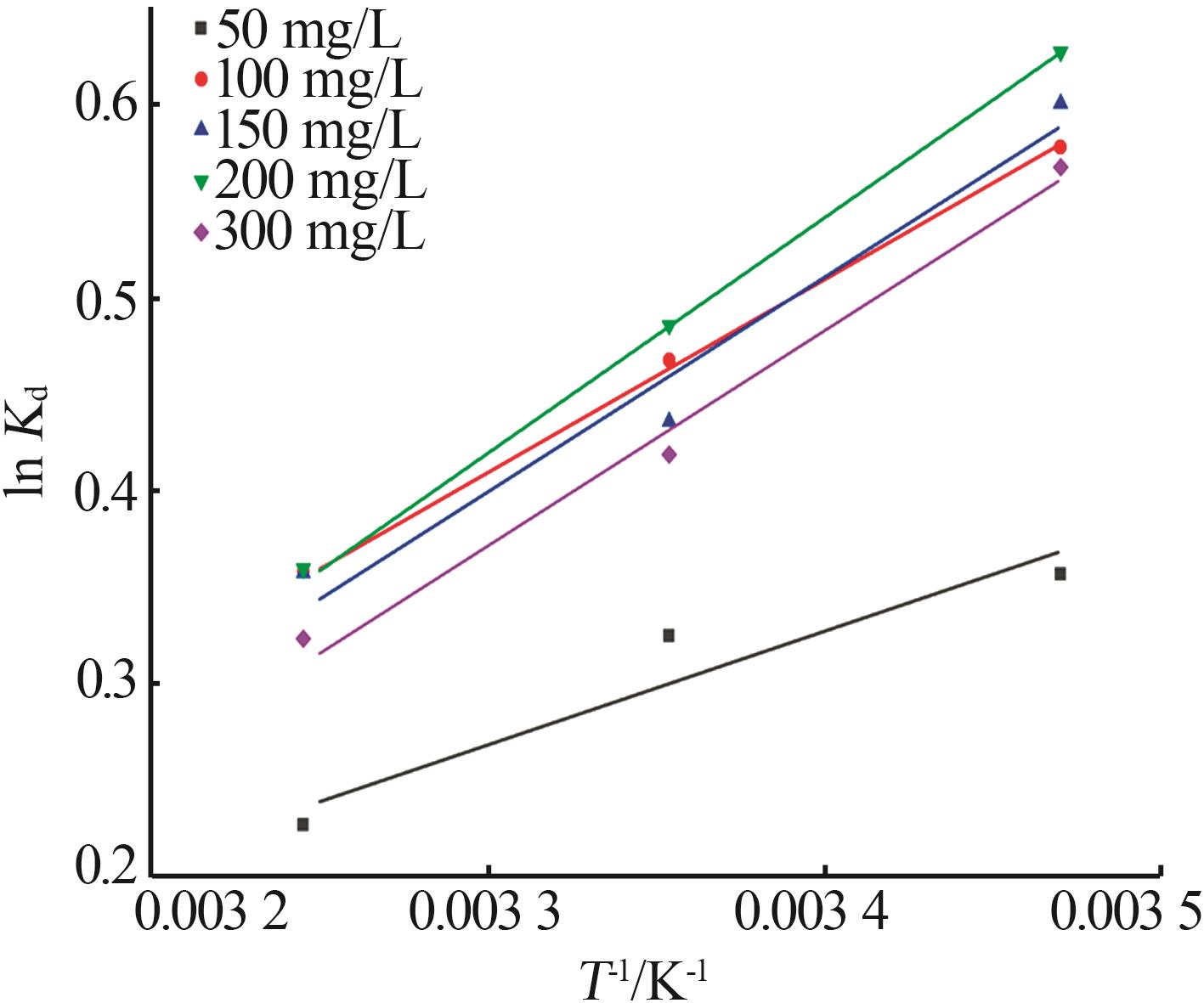
Table 4
Thermodynamic fitting parameters of MIL-88Afor adsorption of As(Ⅲ)"
ρ0 / (mg·L-1) | ΔH/ (kJ·mol-1) | ΔS/ (J·mol-1) | ΔG/(kJ·mol-1) | ||
|---|---|---|---|---|---|
| 288 K | 298 K | 308 K | |||
| 50 | -4.771 1 | -13.492 8 | -0.856 1 | -0.806 4 | -0.582 3 |
| 100 | -8.101 4 | -23.298 1 | -1.385 5 | -1.160 6 | -0.919 1 |
| 150 | -9.011 4 | -26.381 2 | -1.439 7 | -1.081 5 | -0.916 4 |
| 200 | -9.882 6 | -29.086 5 | -1.503 8 | -1.205 0 | -0.922 5 |
| 300 | -9.031 7 | -26.680 5 | -1.361 1 | -1.039 5 | -0.830 0 |
| 1 | ZHANG Liankai, QIN Xiaoqun, TANG Jiansheng,et al.Review of arsenic geochemical characteristics and its significance on arsenic pollution studies in Karst groundwater,Southwest China[J].Applied Geochemistry,2017,77:80-88. |
| 2 | 曹文庚,王妍妍,任宇,等.含砷地下水的治理技术现状与进展[J].中国地质,2022,49(5):1408-1426. |
| CAO Wengeng, WANG Yanyan, REN Yu,et al.Status and progress of treatment technologies for arsenic-bearing groundwater[J].Geology in China,2022,49(5):1408-1426. | |
| 3 | CLANCY T M, HAYES K F, RASKIN L.Arsenic waste management:A critical review of testing and disposal of arsenic-bearing solid wastes generated during arsenic removal from drinking water[J].Environmental Science & Technology,2013,47(19):10799-10812. |
| 4 | WH O.Arsenic in drinking-water:Background document for development of who guidelines for drinking-water quality[J].2003. |
| 5 | BHATTACHARYA P, WELCH A H, AHMED K M,et al.Arsenic in groundwater of sedimentary aquifers[J].Applied Geochemistry,2004,19(2):163-167. |
| 6 | PESSOA LOPES M, GALINHA C F, CRESPO J G,et al.Optimisation of arsenate removal from water by an integrated ion-exchange membrane process coupled with Fe co-precipitation[J].Separation and Purification Technology,2020,246:116894. |
| 7 | GUO Jing, CHENG Jianping, WANG Jiaquan,et al.Simultaneous removal of trivalent arsenic and nitrate using microbial fuel cells[J].Processes,2021,9(4):673. |
| 8 | RIBEIRO I C A, VASQUES I C F, TEODORO J C,et al.Fast and effective arsenic removal from aqueous solutions by a novel low-cost eggshell byproduct[J].Science of the Total Environment,2021,783:147022. |
| 9 | MOHAMAD YUSOF M S, OTHMAN M H D, ABDUL WAHAB R,et al.Effects of pre and post-ozonation on POFA hollow fibre ceramic adsorptive membrane for arsenic removal in water[J].Journal of the Taiwan Institute of Chemical Engineers,2020,110:100-111. |
| 10 | 何智颖,袁君帆,花超,等.从含砷废水中除砷工艺研究进展[J].湿法冶金,2023,42(4):330-334. |
| HE Zhiying, YUAN Junfan, HUA Chao,et al.Research progress on removing of arsenic from arsenic-containing wastewater[J].Hydrometallurgy of China,2023,42(4):330-334. | |
| 11 | WEN Zhipan, LU Jun, ZHANG Yalei,et al.Facile inverse micelle fabrication of magnetic ordered mesoporous iron cerium bimetal oxides with excellent performance for arsenic removal from water[J].Journal of Hazardous Materials,2020,383:121172. |
| 12 | PUROHIT S, CHINI M K, CHAKRABORTY T,et al.Rapid removal of arsenic from water using metal oxide doped recyclable cross-linked chitosan cryogel[J].SN Applied Sciences,2020,2(4):768. |
| 13 | AMEN R, BASHIR H, BIBI I,et al.A critical review on arsenic removal from water using biochar-based sorbents:The significance of modification and redox reactions[J].Chemical Engineering Journal,2020,396:125195. |
| 14 | FOLENS K, LEUS K, NICOMEL N R,et al.Fe3O4@MIL-101-A selective and regenerable adsorbent for the removal of As species from water[J].European Journal of Inorganic Chemistry,2016,2016(27):4395-4401. |
| 15 | HE Xingyu, DENG Fang, SHEN Tingting,et al.Exceptional adsorption of arsenic by zirconium metal-organic frameworks:Engineering exploration and mechanism insight[J].Journal of Colloid and Interface Science,2019,539:223-234. |
| 16 | HUO Jiangbo, XU Lei, YANG J C E,et al.Magnetic responsive Fe3O4-ZIF-8 core-shell composites for efficient removal of As(Ⅲ) from water[J].Colloids and Surfaces A:Physicochemical and Engineering Aspects,2018,539:59-68. |
| 17 | WU Hao, MA Mengdan, GAI Weizhuo,et al.Arsenic removal from water by metal-organic framework MIL-88A microrods[J].Environmental Science and Pollution Research,2018,25(27):27196-27202. |
| 18 | FU Huifen, SONG Xiaoxu, WU Lin,et al.Room-temperature preparation of MIL-88A as a heterogeneous photo-Fenton catalyst for degradation of rhodamine B and bisphenol a under visible light[J].Materials Research Bulletin,2020,125:110806. |
| 19 | LI Xiyi, PI Yunhong, WU Liqiong,et al.Facilitation of the visible light-induced Fenton-like excitation of H2O2 via heterojunction of g-C3N4/NH2-Iron terephthalate metal-organic framework for MB degradation[J].Applied Catalysis B:Environmental,2017,202:653-663. |
| 20 | YANG Jichun, YIN Xuebo.CoFe2O4@MIL-100(Fe) hybrid magnetic nanoparticles exhibit fast and selective adsorption of arsenic with high adsorption capacity[J].Scientific Reports,2017,7:40955. |
| 21 | SIMONIN J P.On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics[J].Chemical Engineering Journal,2016,300:254-263. |
| 22 | EZZATI R.Derivation of pseudo-first-order,pseudo-second-order and modified pseudo-first-order rate equations from Langmuir and Freundlich isotherms for adsorption[J].Chemical Engineering Journal,2020,392:123705. |
| 23 | NASIR A M, NORDIN N A H MD, GOH P S,et al.Application of two-dimensional leaf-shaped zeolitic imidazolate framework (2D ZIF-L) as arsenite adsorbent:Kinetic,isotherm and mechani-sm[J].Journal of Molecular Liquids,2018,250:269-277. |
| 24 | JIAN Meipeng, WANG Huan, LIU Ruiping,et al.Self-assembled one-dimensional MnO2@zeolitic imidazolate framework-8 nanostructures for highly efficient arsenite removal[J].Environmental Science:Nano,2016,3(5):1186-1194. |
| 25 | BRANCA C, D'ANGELO G, CRUPI C,et al.Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions:A FTIR-ATR study on chitosan and chitosan/clay films[J].Polymer,2016,99:614-622. |
| [1] | LIU Zihan, XI Guojun, LEI Guangping. Application of MOFs in adsorption refrigeration/heat pump [J]. Inorganic Chemicals Industry, 2023, 55(4): 20-26. |
| [2] | WEI Fengdan, ZHOU Huan, XIA Panping, ZHAO Yun. Boron species transformation and distribution law of Mg(BO2)2decomposed in LiCl aqueous solution [J]. Inorganic Chemicals Industry, 2023, 55(4): 45-53. |
| [3] | NAN Jinjian,WU Suli. Preparation of uniform cadmium sulfide microspheres in aqueous solution and their application in structural color [J]. Inorganic Chemicals Industry, 2022, 54(4): 94-99. |
| [4] | Shen Wei,Wang Sinan,Liang Xuemei,Wei Jinyun,Pan Yujie,Nong Tiantian,Zhou Yan,Tan Xuecai,Huang Zaiyin. Research progress of nano MOFs and their derivatives for supercapacitors [J]. Inorganic Chemicals Industry, 2021, 53(6): 79-86. |
| [5] | WANG Tian-Gui, HU Dong-Fang, LI Qiang. Leaching of hexavalent chromium contained in solid waste from new production process of potassium chromate using salt solutions [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(8): 66-. |
| [6] | JIANG Xiang-Fei, LI Jun, LIU Xue-Feng, LUO Jian-Hong, JIN Yang, YAO Wei-Dong. Study on purification of yellow phosphorus by nitric acid oxidation combined with zone melting [J]. INORGANICCHEMICALSINDUSTRY, 2014, 46(3): 32-. |
| [7] | MA Hang, YANG Ya-Bin, LIANG Xue-Song. Method of removing arsenic from polyphosphoric acid by hydrochloric acid [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(11): 33-. |
| [8] | Liu Houfan;Pan Qinghui;Zhou Xinmu;Xia Guoqiang;Gao Changhua. Study on arsenic removal in the preparation of feed grade zinc oxide by ammonia-ammonium sulfate method [J]. INORGANICCHEMICALSINDUSTRY, 2008, 0(3): 0-0. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||