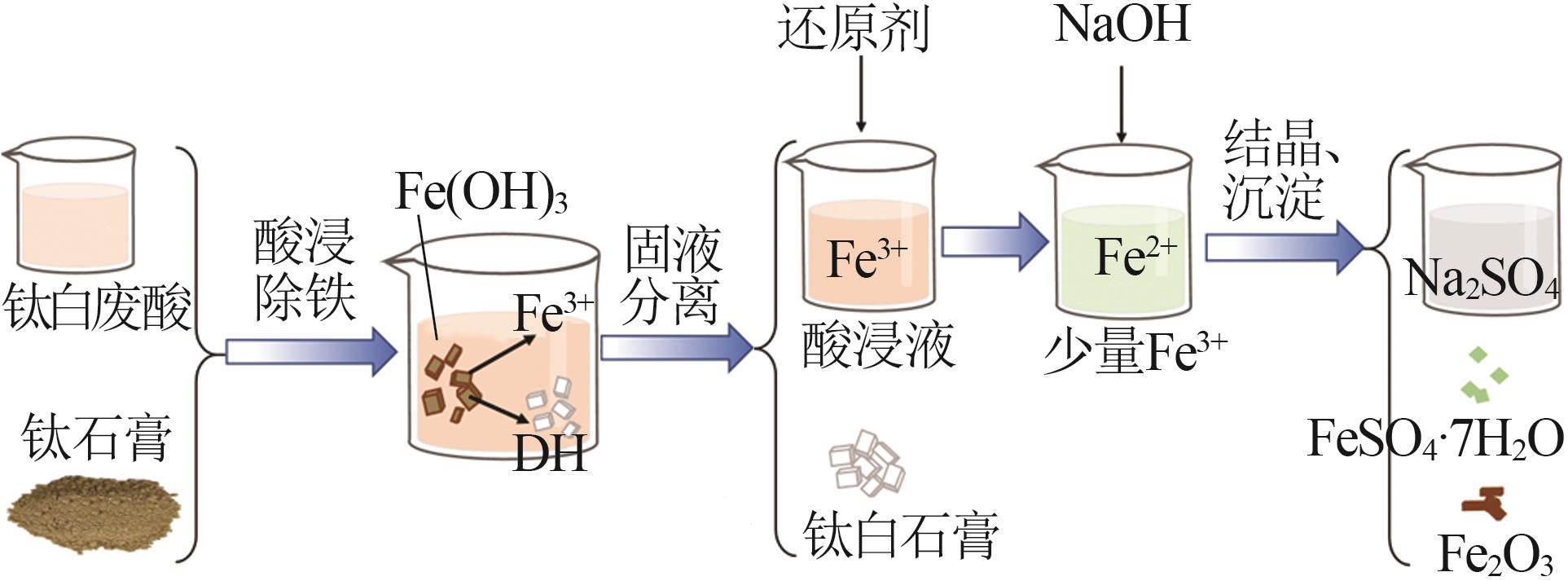
Inorganic Chemicals Industry ›› 2024, Vol. 56 ›› Issue (1): 114-120.doi: 10.19964/j.issn.1006-4990.2023-0141
• Environment·Health·Safety • Previous Articles Next Articles
Study on kinetic of iron leaching from titanium gypsum and its cyclic acid leaching process
XIANG Mengqi1( ), MENG Hua2(
), MENG Hua2( ), WANG Ye1, MENG Xianzhang3, BAI Yuhang1, WANG Yujunyao1, ZHANG Yidan1
), WANG Ye1, MENG Xianzhang3, BAI Yuhang1, WANG Yujunyao1, ZHANG Yidan1
- 1. College of Chemical Engineering,Sichuan University,Chengdu 610065,China
2. Chongqing Vocational College of Chemical Technology,Chongqing 400020,China
3. Panzhihua Steel Metallurgical Materials Co.,Ltd.,Panzhihua 617023,China
-
Received:2023-03-14Online:2024-01-10Published:2024-01-18 -
Contact:MENG Hua E-mail:1131638127@qq.com;mhua08@163.com
CLC Number:
Cite this article
XIANG Mengqi, MENG Hua, WANG Ye, MENG Xianzhang, BAI Yuhang, WANG Yujunyao, ZHANG Yidan. Study on kinetic of iron leaching from titanium gypsum and its cyclic acid leaching process[J]. Inorganic Chemicals Industry, 2024, 56(1): 114-120.
share this article
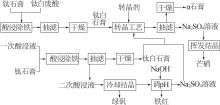
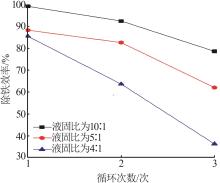
Table 1
Common application directions and characteristics of titanium gypsum"
| 应用方向 | 优点 | 缺点 | |
|---|---|---|---|
不除铁 利用 | 水泥缓释剂 | 成本低,现今主要消纳方式 | 附加值低,水泥掺量有限(5%) |
| 路基材料 | 成本低,消纳较大 | 暂无标准,水泥掺量有限(10%) | |
| 复合胶凝材料 | 成本低,用量大 | 力学性能稍显不足 | |
| 土壤改性剂 | 成本低 | 附加值低 | |
| 生态复合肥 | 成本低 | 附加值较高,技术尚不成熟 | |
除铁 利用 | 二水β石膏 | 可在废酸中和时分级制备 | 附加值一般 |
| 半水β石膏 | 附加值提高,可作胶凝材料 | 除铁、烘干成本有所增加 | |
| 半水α石膏 | 附加值高,可作建筑材料 | 除铁、转晶成本较高 | |
| 石膏晶须 | 附加值高,可作增强耐磨材料 | 除铁、转晶成本较高 |
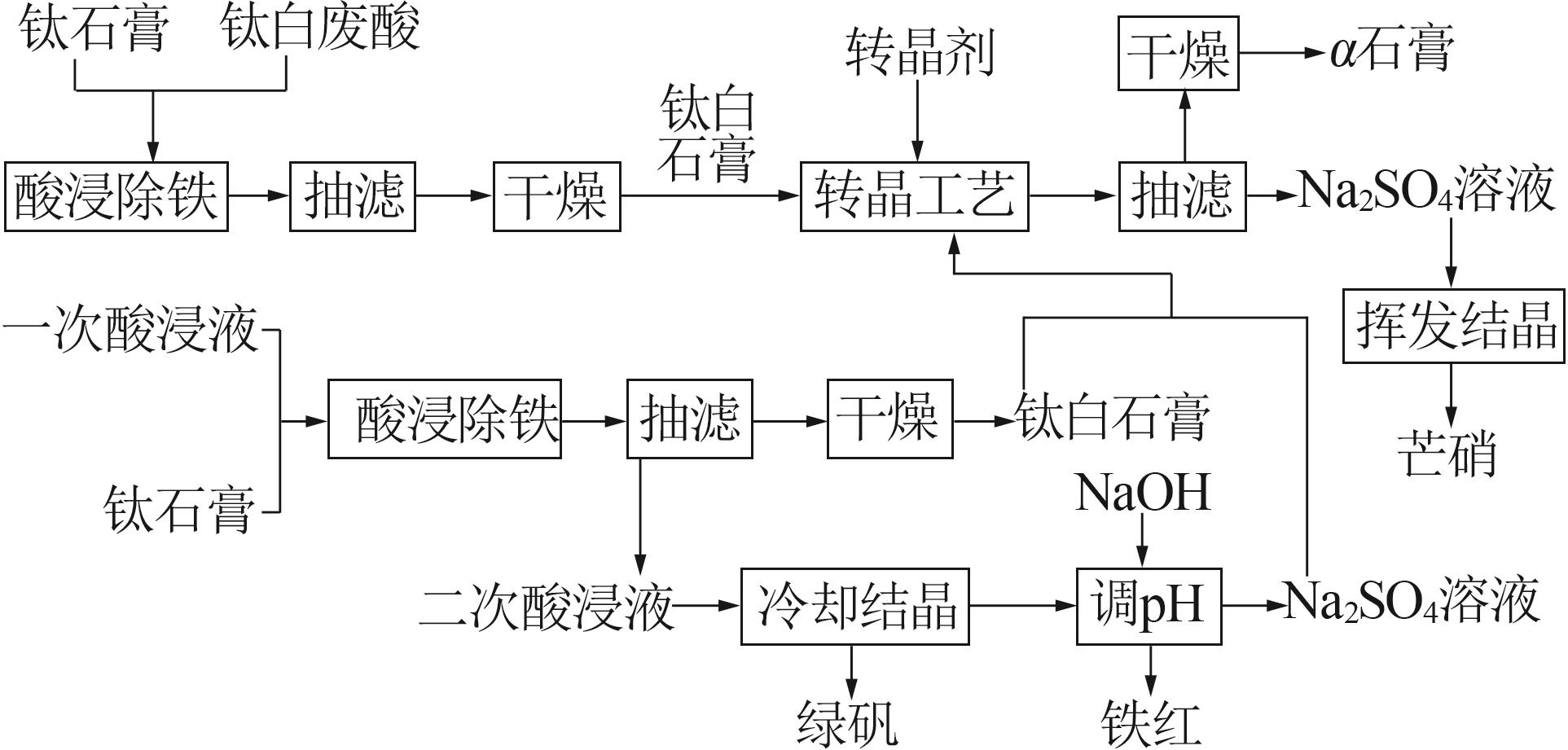
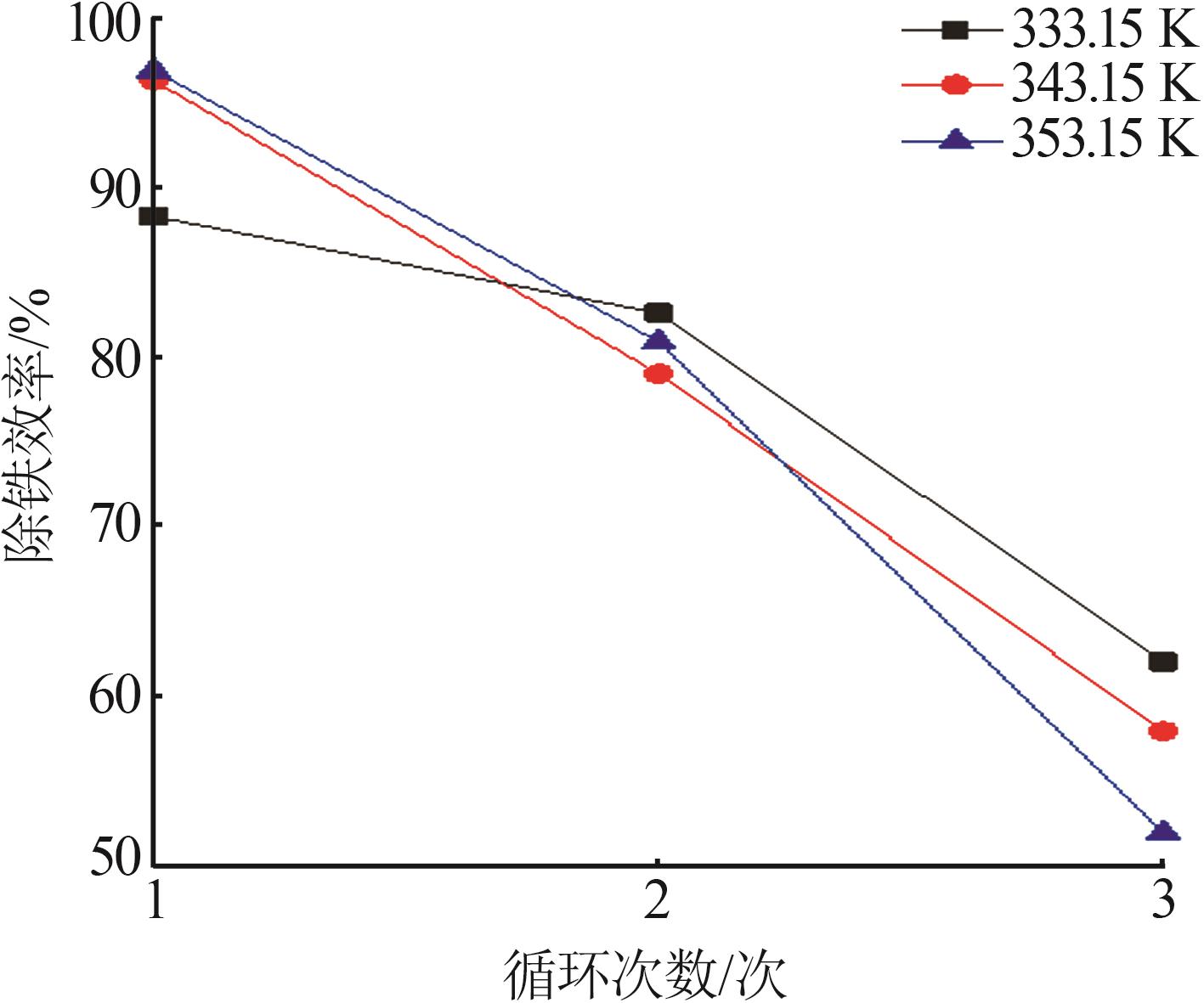
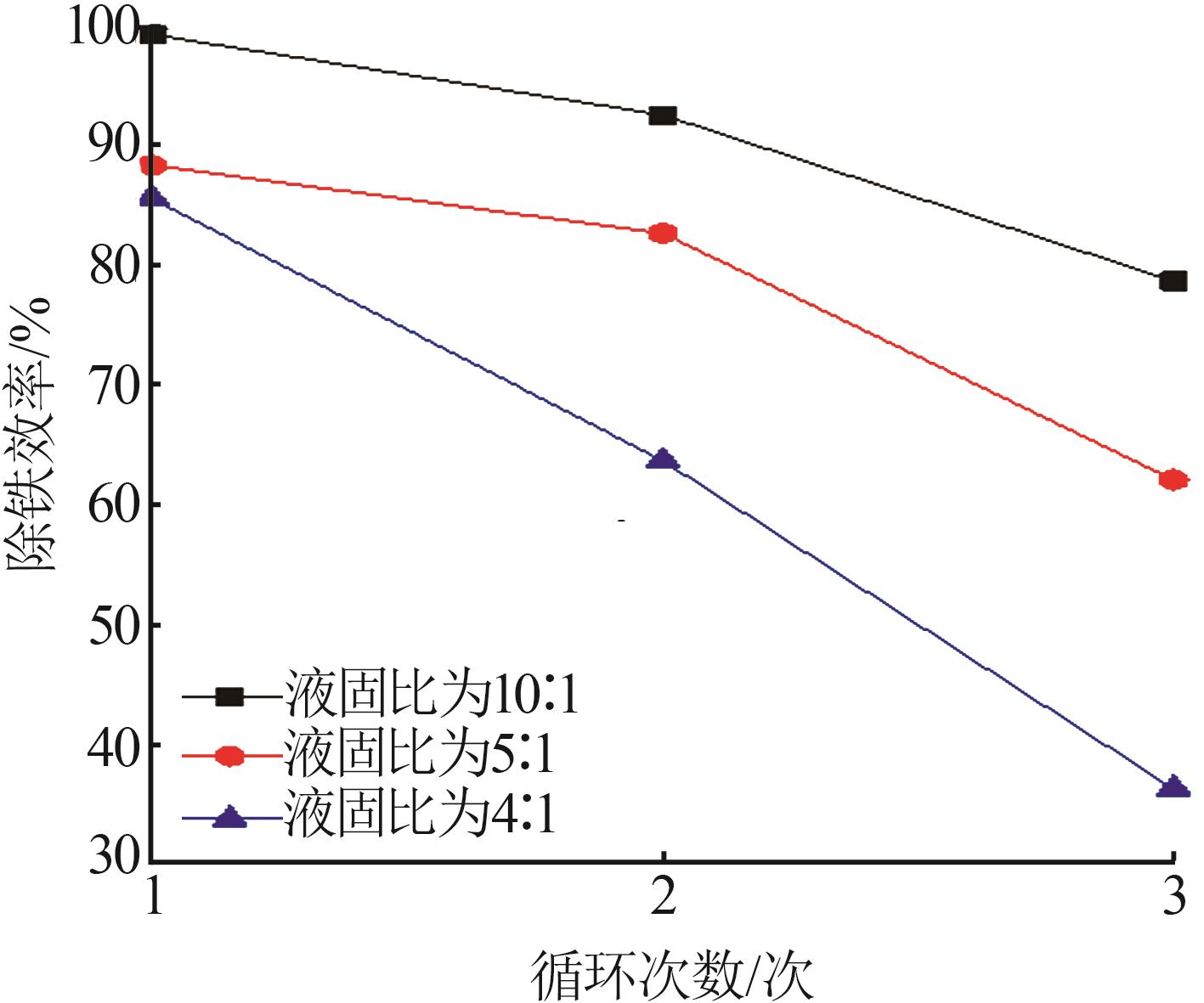
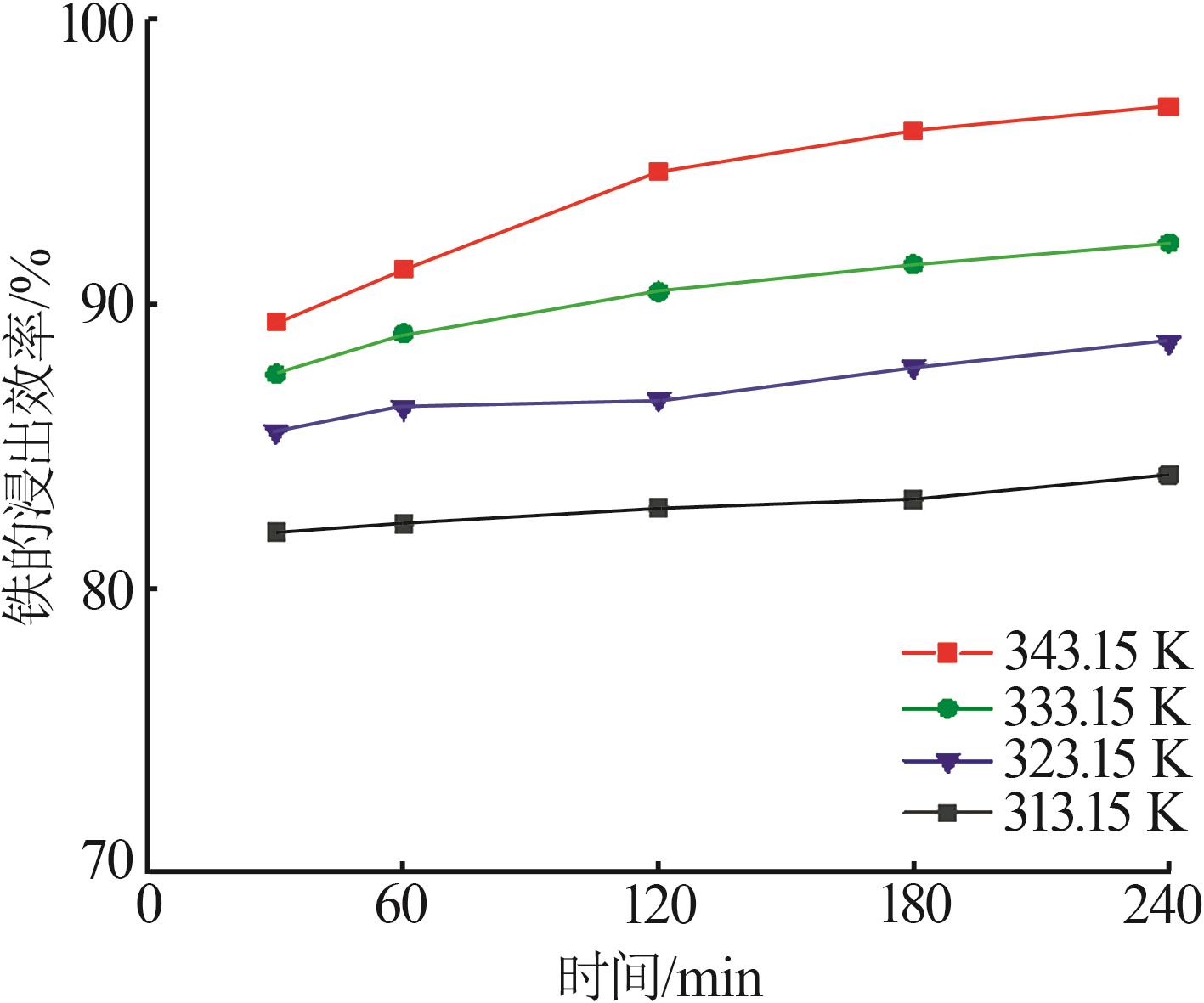
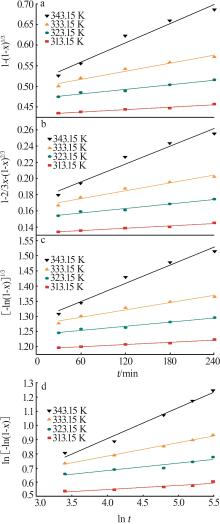
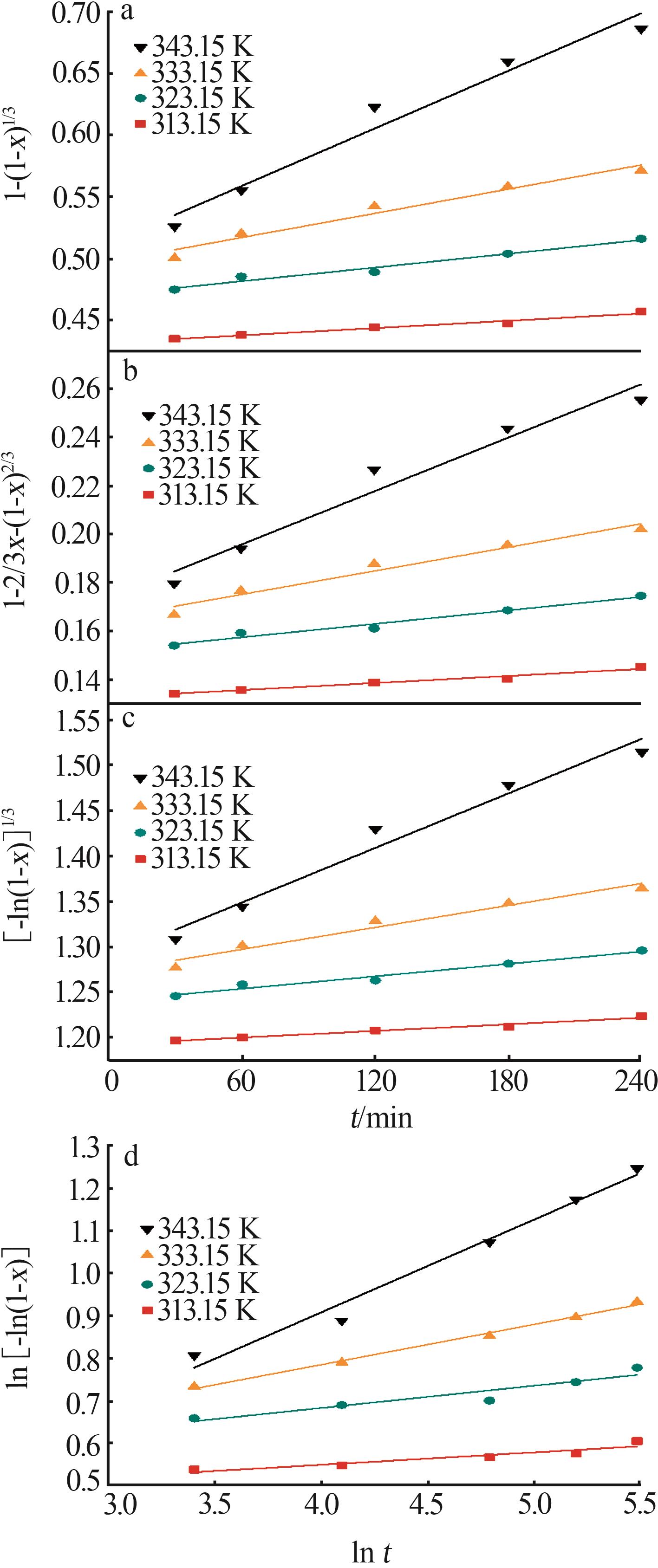
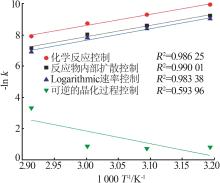
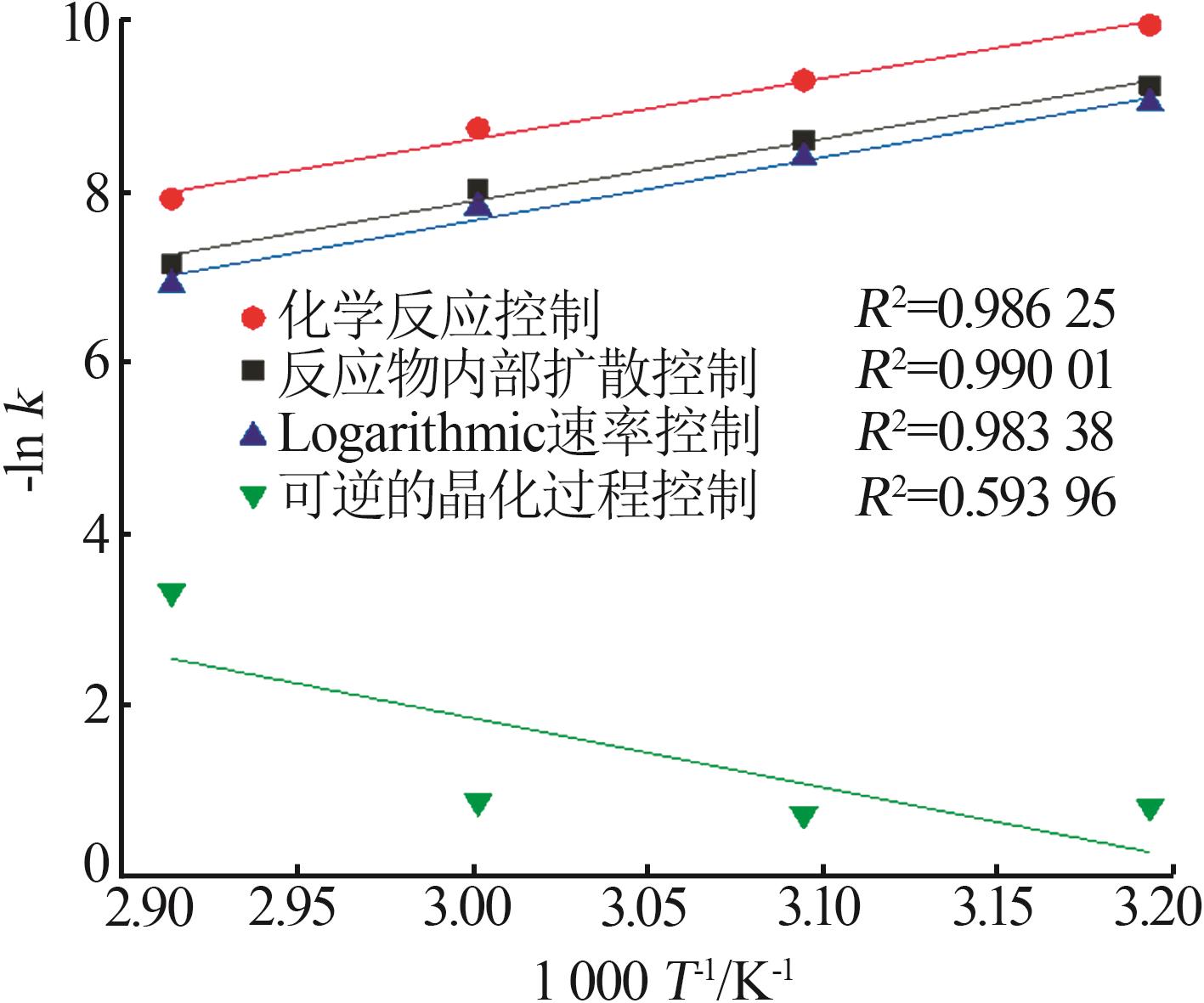
Table 3
Fitting parameters for leaching kinetics of iron in titanium waste acid leachate"
| T/K | 化学反应控制 | 反应物内部扩散控制 | Logarithmic 速率控制 | 可逆的晶化过程控制 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ln k | R2 | ln k | R2 | ln k | R2 | ln k | R2 | |||||
| 313.15 | 0.974 32 | 9.225 80 | 0.974 02 | 9.930 96 | 0.974 47 | 9.016 36 | 0.883 53 | 0.836 95 | ||||
| 323.15 | 0.970 86 | 8.592 85 | 0.970 87 | 9.286 53 | 0.970 84 | 8.387 70 | 0.893 82 | 0.747 43 | ||||
| 333.15 | 0.965 31 | 8.026 98 | 0.964 25 | 8.727 23 | 0.966 35 | 7.813 90 | 0.994 09 | 0.900 54 | ||||
| 343.15 | 0.966 89 | 7.159 67 | 0.960 31 | 7.908 44 | 0.971 89 | 6.907 76 | 0.980 07 | 3.340 20 | ||||
| 1 | 李逸晨.JC/T 2625—2021《钛石膏》标准分析[J].硫酸工业,2022(4):7-11. |
| LI Yichen.Standard analysis of JC/T 2625-2021 Titanium gyps-um[J].Sulphuric Acid Industry,2022(4):7-11. | |
| 2 | 付一江.工业副产石膏—钛石膏的现状及综合利用前景[J].钢铁钒钛,2019,40(6):63-66,100. |
| FU Yijiang.Situation and comprehensive utilization prospect of titanium gypsum[J].Iron Steel Vanadium Titanium,2019,40(6):63-66,100. | |
| 3 | 龚家竹.钛石膏与磷石膏固废耦合资源化利用技术进展[J].无机盐工业,2019,51(1):1-6,11. |
| GONG Jiazhu.Progress in coupling utilization technology of titanium gypsum and phosphogypsum solid waste[J].Inorganic Che-Industry micals,2019,51(1):1-6,11. | |
| 4 | 肖世玉,吕淑珍,宁美,等.富铁钛石膏做水泥缓凝剂的试验研究[J].混凝土与水泥制品,2016(12):82-87. |
| XIAO Shiyu, Shuzhen LÜ, NING Mei,et al.Experimental research on cement retarder by titanium gypsum with iron in rich[J].China Concrete and Cement Products,2016(12):82-87. | |
| 5 | ZHA Fusheng, QIAO Borui, KANG Bo,et al.Engineering properties of expansive soil stabilized by physically amended titanium gypsum[J].Construction and Building Materials,2021,303:124456. |
| 6 | 黄绪泉,刘立明,别双桥,等.钛石膏改性胶凝材料制备及水化机理[J].三峡大学学报(自然科学版),2016,38(1):45-50. |
| HUANG Xuquan, LIU Liming, BIE Shuangqiao,et al.Preparation and hydration mechanism of modified titanium gypsum cementing materials[J].Journal of China Three Gorges University(Natural Sciences),2016,38(1):45-50. | |
| 7 | 邹丽娜,徐婧婧,陈铮铮,等.水旱轮作下钛石膏对土壤砷铅有效性的影响研究[J].农业环境科学学报,2021,40(4):774- 781. |
| ZOU Lina, XU Jingjing, CHEN Zhengzheng,et al.Effect of titanium gypsum on the availability of arsenic and lead in agricultural soil under paddy-dryland rotation conditions[J].Journal of Agro-Environment Science,2021,40(4):774-781. | |
| 8 | 汪初雷,任浩荣,刘德智,等.钛石膏除杂制备高强石膏及回收铁的工艺[J].化工矿物与加工,2022,51(5):44-48. |
| WANG Chulei, REN Haorong, LIU Dezhi,et al.Preparation of high strength gypsum and iron recovery by removal of impurities from titanium gypsum[J].Industrial Minerals & Processing,2022,51(5):44-48. | |
| 9 | 杨贺,陈伟,梁贺之,等.钛石膏强度与含水率预测模型建立与强度机理分析[J].钢铁钒钛,2020,41(5):107-112. |
| YANG He, CHEN Wei, LIANG Hezhi,et al.Titanium gypsum strength transition point model establishment and strength mechanism analysis[J].Iron Steel Vanadium Titanium,2020,41(5):107-112. | |
| 10 | 邵国庆.钛石膏酸浸除杂试验研究[J].钢铁钒钛,2020,41(3):90-94. |
| SHAO Guoqing.Experimental study on acid leaching purification of titanium gypsum[J].Iron Steel Vanadium Titanium,2020,41(3):90-94. | |
| 11 | 蒋美雪,孙红娟,彭同江.钛石膏硫酸浸取法提取铁质氧化物及石膏的物相变化[J].化工进展,2019,38(4):2030-2036. |
| JIANG Meixue, SUN Hongjuan, PENG Tongjiang.Extraction of iron oxide from titanium gypsum by sulfuric acid leaching and phase transform of gypsum[J].Chemical Industry and Engineering Progress,2019,38(4):2030-2036. | |
| 12 | 陈书锐,杨绍利,马兰,等.盐酸浸出钛石膏实验研究[J].无机盐工业,2020,52(2):65-68. |
| CHEN Shurui, YANG Shaoli, MA Lan,et al.Study on leaching of titanium gypsum with hydrochloric acid[J].Inorganic Chemicals Industry,2020,52(2):65-68. | |
| 13 | CHEN Qiuju, DING Wenjin, SUN Hongjuan,et al.Synthesis of anhydrite from red gypsum and acidic wastewater treatment[J].Journal of Cleaner Production,2021,278:124026. |
| 14 | 李根,石海信,冯洋洋,等.钢铁酸洗废液铁系颜料制备及改性技术研究进展[J].化学工程师,2022,36(7):68-72. |
| LI Gen, SHI Haixin, FENG Yangyang,et al.Research progress in preparation and modification of iron-based pigments from waste liquor of iron and steel pickling[J].Chemical Engineer,2022,36(7):68-72. | |
| 15 | LI Xianbo, HE Yuqi.Preparation of α-hemihydrate gypsum from phosphogypsum in glycerol and Na2SO4 mixed solutions under atmospheric pressure[J].Journal of Crystal Growth,2021,568-569:126184. |
| 16 | CHEN Gaoxiang, LIU Ruicun, SONG Chun,et al.Preparation of hemihydrate gypsum with controllable morphology in Glycerol-NaCl-Water solutions with maleic acid[J].Journal of Crystal Growth,2021,576:126360. |
| 17 | LIN Yan, SUN Hongjuan, PENG Tongjiang,et al.The leaching kinetics of iron from titanium gypsum in a citric acid medium and obtain materials by leaching liquid[J].Molecules,2023,28(3): 952. |
| [1] | TIAN Peng, ZHOU Ruohui, XU Qianjin, LIU Kunji, PANG Hongchang, NING Guiling. Synthesis and dehydration dynamics of boehmite microcrystalline with different particle sizes [J]. Inorganic Chemicals Industry, 2023, 55(11): 27-36. |
| [2] | WEI Kang, ZHANG Hao, GAN Shunpeng. Research on mechanical drive enhancement crystallization process for titanium gypsum [J]. Inorganic Chemicals Industry, 2023, 55(11): 78-85. |
| [3] | MA Lei,SHENG Yu,ZHOU Junhong,YANG Yujun,LUO Hui,PAN Chunying,WANG Guo. Study on new process of comprehensive utilization and separation of sulfur and calcium of titanium gypsum [J]. Inorganic Chemicals Industry, 2022, 54(7): 124-128. |
| [4] | WANG Youyou,YUAN Hao,HAN Qingqing,CHEN Shiying. Activation of activator on fly ash?titanium gypsum?calcium carbide slag system and its hydration mechanism [J]. Inorganic Chemicals Industry, 2022, 54(6): 115-119. |
| [5] | ZHANG Shaokang,ZHAO Hua,LIU Runjing. Study on macro?kinetics of carbonization reaction of ammonia alkali salt sludge [J]. Inorganic Chemicals Industry, 2022, 54(6): 120-124. |
| [6] | ZHANG Shaokang,ZHAO Hua,LIU Runjing. Study on macro?kinetics of carbonization reaction of ammonia alkali salt sludge [J]. Inorganic Chemicals Industry, 2022, 54(6): 120-124. |
| [7] | Meng Hua,Zhao Jingyi,Nie Chaoyang,Wang Ye. Study on simulation process of reducing titanium dioxide gypsum by sulfur fluidization [J]. Inorganic Chemicals Industry, 2021, 53(9): 61-66. |
| [8] | Yang Xiaohong,Xue Xishi,Zhang Lulu,Chang Jun. Kinetics study of calcium leaching from electrolytic manganese residue by hydrochloric acid [J]. Inorganic Chemicals Industry, 2021, 53(1): 82-86. |
| [9] | Chen Shurui,Yang Shaoli,Ma Lan,Hou Jing. Study on leaching of titanium gypsum with hydrochloric acid [J]. Inorganic Chemicals Industry, 2020, 52(2): 65-68. |
| [10] | Ding Ke1,Sun Xiaojun1,Li Huijie1,Huang Nana1,Qiu Long1,Liu Yunyi1,2,Li Xue1,2. Study on kinetics and mechanism of ammonia evaporation reaction of magnesium oxide [J]. Inorganic Chemicals Industry, 2019, 51(11): 31-35. |
| [11] | MA Guang-Qiang, ZOU Min, XIA Dong. Experimental study on titanium concentrate leaching to prepare Ti-rich material with titanium dioxide waste acid [J]. INORGANICCHEMICALSINDUSTRY, 2016, 48(8): 67-. |
| [12] | CUI Yi-Shun- , XIANG Yun-Gang. Study on sulfuric acid leaching kinetics of pyrolusite with ferrous sulfate as reducing agent [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(1): 26-. |
| [13] | YU Xiao-Fei, BAO Xin-Xia, LI Qi-Ming, LI Fang, CHEN Ping. Preparation of Al2O3 supported CoB amorphous alloy catalyst and its application in hydrogen generation [J]. INORGANICCHEMICALSINDUSTRY, 2015, 47(1): 59-. |
| [14] | WANG Ben, MA Xiang, ZHANG Shu-Juan, JIANG Jie, SHI Ning. Analysis on thermal risk of hydrogen peroxide [J]. INORGANICCHEMICALSINDUSTRY, 2013, 45(3): 15-. |
| [15] | GOU Ping, FU Quan-Jun, WANG Xin-Long, YANG Lin, FU Yu-Xin, ZHANG Zhi-Ye. Research on double decomposition reaction kinetics of potassium chloride and phosphoric acid [J]. INORGANICCHEMICALSINDUSTRY, 2012, 44(4): 13-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
|
||