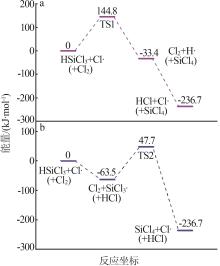
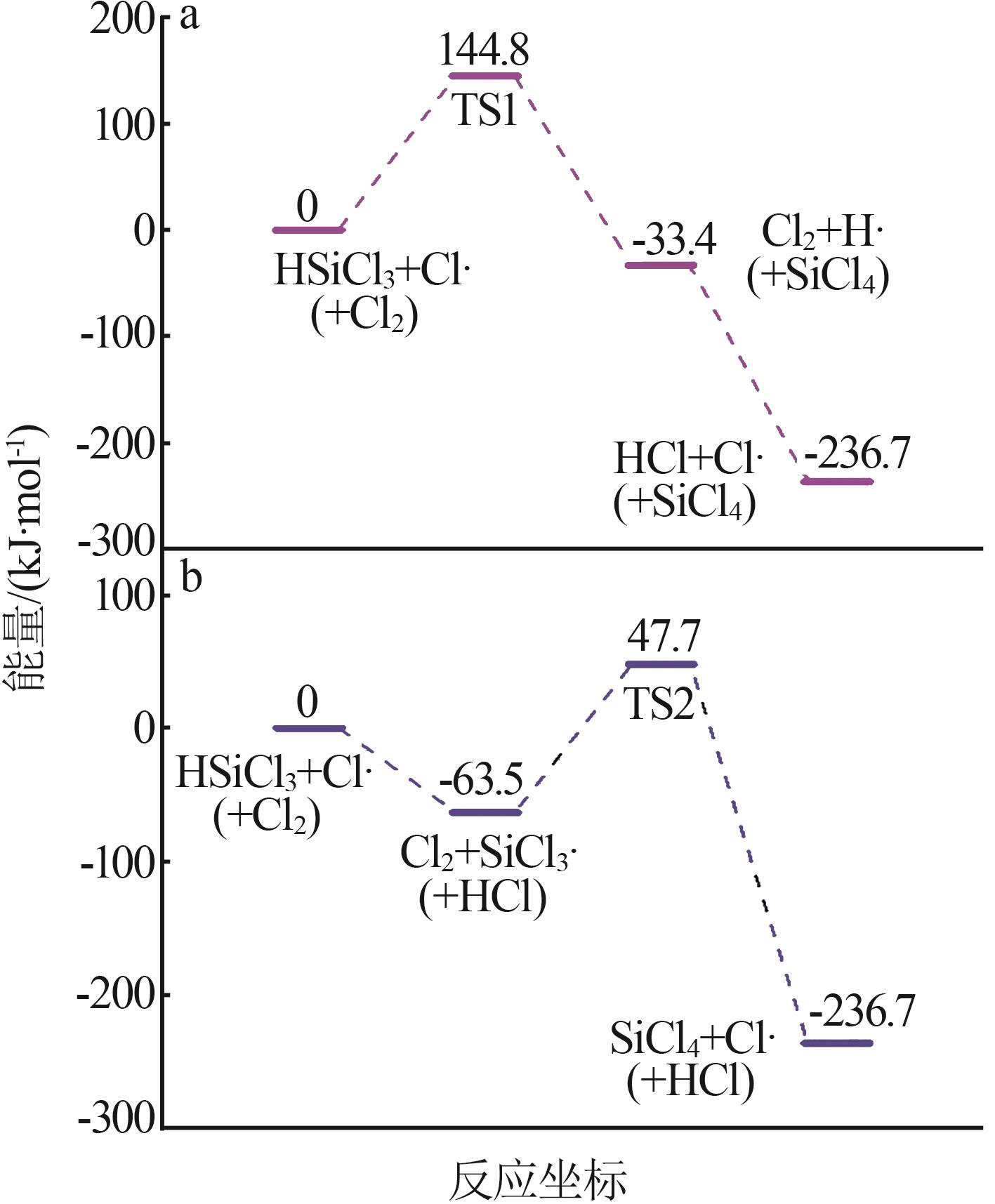
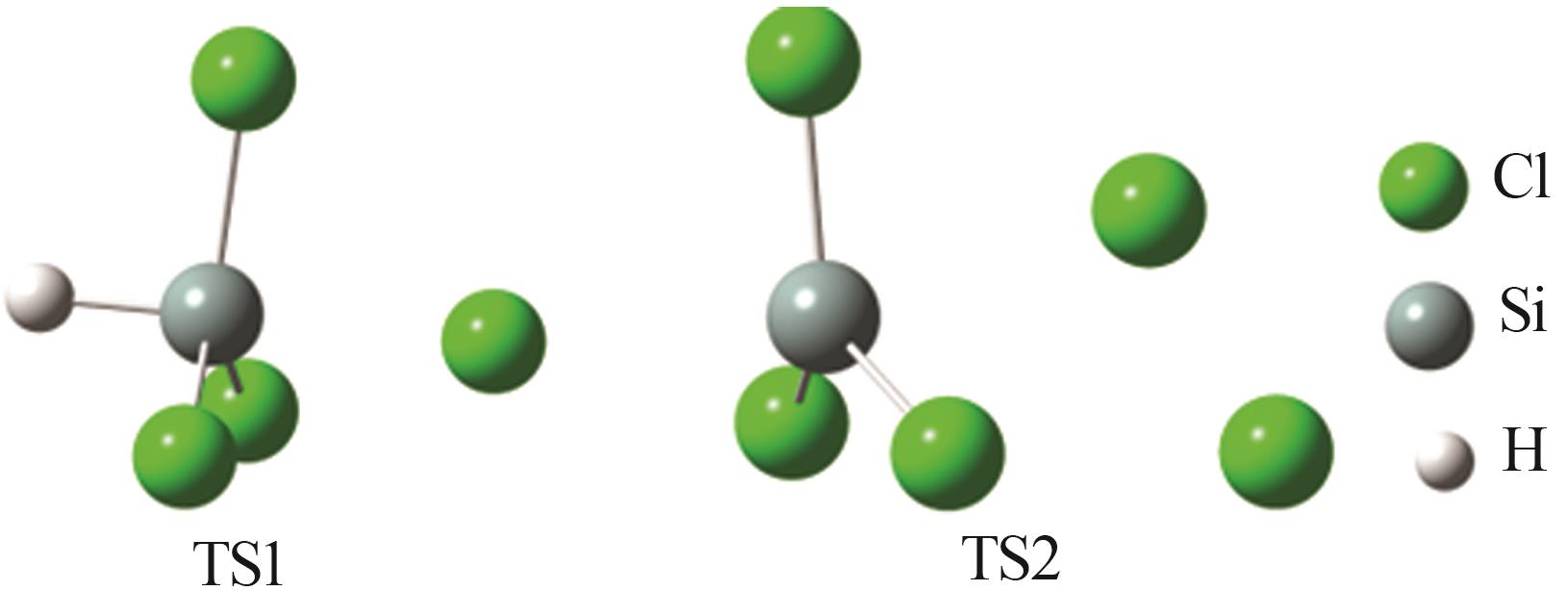
| 1 |
CHIGONDO F. From metallurgical-grade to solar-grade silicon:An overview[J]. Silicon, 2018, 10(3):789-798.
doi: 10.1007/s12633-016-9532-7
|
| 2 |
RANJAN S, BALAJI S, PANELLA R A, et al. Silicon solar cell production[J]. Computers & Chemical Engineering, 2011, 35(8):1439-1453.
doi: 10.1016/j.compchemeng.2011.04.017
|
| 3 |
王虎虎, 彭文才, 张建树, 等. 四氯化硅氢化关键反应的密度泛函理论模拟研究[J]. 石河子大学学报(自然科学版), 2021, 39(5):537-540.
|
|
WANG Huhu, PENG Wencai, ZHANG Jianshu, et al. The research of key reaction of SiCl4 hydrogenation with density functional theory simulation[J]. Journal of Shihezi University(Natural Science), 2021, 39(5):537-540.
|
| 4 |
黄国强, 张明鑫. 一种去除氯硅烷中含碳杂质的反应精馏提纯方法及装置: 中国, 110980742A[P]. 2020-04-10.
|
| 5 |
王红星, 陈锦溢, 华超, 等. 一种吸附脱除甲基氯硅烷杂质制备高纯三氯氢硅的装置和方法: 中国, 109205627A[P]. 2019-01- 15.
|
| 6 |
杨典, 王芳, 万烨, 等. 分离三氯氢硅中甲基二氯硅烷的研究进展[J]. 无机盐工业, 2021, 53(3):30-33,72.
|
|
YANG Dian, WANG Fang, WAN Ye, et al. Research progress on separation process of methyl dichlorosilane from trichlorsilane[J]. Inorganic Chemicals Industry, 2021, 53(3):30-33,72.
|
| 7 |
杨典, 万烨, 赵雄, 等. 用于高纯氯硅烷生产中去除含碳杂质的方法及装置: 中国, 111115637B[P]. 2023-08-11.
|
| 8 |
万烨, 肖劲, 严大洲, 等. 光氯化反应脱除三氯氢硅中的甲基二氯硅烷[J]. 精细化工, 2020, 37(1):201-206,216.
|
|
WAN Ye, XIAO Jin, YAN Dazhou, et al. Removal of methyldichlorosilane from trichlorosilane via photochemical chlorination[J]. Fine Chemicals, 2020, 37(1):201-206,216.
|
| 9 |
张明鑫, 黄国强. 氯硅烷中甲基二氯硅烷的转化反应工艺[J]. 精细化工, 2020, 37(4):758-764.
|
|
ZHANG Mingxin, HUANG Guoqiang. Conversion reaction process of methyldichlorosilane in chlorosilane[J]. Fine Chemicals, 2020, 37(4):758-764.
|
| 10 |
GENG Qiang, HUANG Guoqiang. Catalytic conversion of carbon-containing impurity methyldichlorosilane to purify raw material trichlorosilane of polysilicon production[J]. Reaction Chemistry & Engineering, 2022, 7(7):1544-1554.
|
| 11 |
ISHIDA M, SAITO H, HASEGAWA M. Method for producing trichlorosilane: US, 20170369325[P]. 2017-12-28.
|
| 12 |
王红星, 陈锦溢, 华超, 等. 一种反应精馏去除三氯氢硅中甲基二氯硅烷的装置和方法: 中国, 109179426A[P]. 2019-01-11.
|
| 13 |
毛威, 苏小平, 王铁艳, 等. 光化法去除四氯化硅中三氯氢硅的实验研究[J]. 无机盐工业, 2010, 42(12):37-39.
|
|
MAO Wei, SU Xiaoping, WANG Tieyan, et al. Experimental study on removing of trichlorosilane in silicon tetrachloride with photochemistry reaction[J]. Inorganic Chemicals Industry, 2010, 42(12):37-39.
|
| 14 |
王艳坤. HSC Chemistry软件在高校化学科研中的应用[J]. 河南教育学院学报(自然科学版), 2013, 22(2):28-30.
|
|
WANG Yankun. Application of HSC chemistry software in university chemical scientific research[J]. Journal of Henan Institute of Education(Natural Science Edition), 2013, 22(2):28-30.
|
| 15 |
GONZALEZ C, SCHLEGEL H B. Reaction path following in mass-weighted internal coordinates[J]. The Journal of Physical Chemistry, 1990, 94(14):5523-5527.
doi: 10.1021/j100377a021
|
| 16 |
DESAIN J D, VALACHOVIC L, JUSINSKI L E, et al. Reaction of chlorine atom with trichlorosilane from 296 to 473 K[J]. The Journal of Chemical Physics, 2006, 125(22):224308.
doi: 10.1063/1.2404673
|
 ), JIA Zhaohang, XU Maolan, MA Tianyue, PENG Wencai(
), JIA Zhaohang, XU Maolan, MA Tianyue, PENG Wencai( ), ZHANG Jianshu(
), ZHANG Jianshu( )
)